Photo AI
Last Updated Sep 24, 2025
Introduction to Solutions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Introduction to Solutions quickly and effectively.
347+ students studying
Introduction to Solutions
Solutions are integral to various sectors, such as pharmaceuticals, where solutions like saline enhance drug delivery by dissolving active components. In food chemistry, sugar solutions affect candy texture and sweetness, while cleaning agents such as detergents effectively remove dirt by dissolving it. Solutions improve solubility and influence reaction rates.
Definition of Solutions
- Solution: Homogeneous mixture with uniformly distributed components.
- Comparison with Other Mixtures:
- Suspensions: Heterogeneous mixtures containing visible particles that settle over time.
- Colloids: Mixtures with particles that do not settle, causing the Tyndall effect. Examples include fog, milk, gels, and aerosols.
Key Differences:
- Solutions: Even distribution, particles do not settle.
- Suspensions: Visible particles, settle over time.
- Colloids: Particles do not settle and scatter light.
Composition of Solutions
Solute and Solvent
- Solute: Substance that is being dissolved. Example: Salt in water.
- Solvent: Medium where solute is dissolved. Example: Water in a sugar solution.
Characteristics
- Effects of Temperature and Pressure:
- Sugar dissolves more rapidly in hot water than cold due to increased molecular motion, enhancing solubility.
- Increased pressure enhances the solubility of gases in liquids, which is relevant for scuba divers as they absorb more nitrogen underwater.
- Fact: Heating increases solubility by supplying energy to break intermolecular forces.
Molecular-Level Interaction
- Interactions and Solubility: Solutes and solvents engage at a molecular level via forces like hydrogen bonding and dipole-dipole interactions. Stronger forces result in better dissolving capacity, forming consistent solutions.
Properties of Solutions
Clarity and Homogeneity
-
Solution: A homogeneous mixture notable for its uniform distribution.
- Solute particles are evenly dispersed within the solvent, such as in saltwater, where the salt disperses evenly ensuring consistent taste.
-
Light Scattering & Particle Size:
- Solutions maintain clarity owing to small solute particles allowing light to pass without scattering.
-
Mixture Differences:
| Mixture Type | Particle Size | Light Interaction |
|---|---|---|
| Solution | Molecular Size | Does not scatter light |
| Colloid | Larger | Scatters light, appears cloudy |
| Suspension | Largest | Scatters light and settles |
This table assists in distinguishing mixtures based on particle size and light interaction, useful for predicting mixture behaviour in experiments.
Stability
-
Definition & Explanation:
- Stability refers to maintaining a uniform distribution without separation under varying conditions, crucial for consistent products such as tea.
-
Factors Influencing Stability:
- Temperature and concentration significantly influence solubility and potential crystallisation.
- Solvent Properties have an impact on stability based on chemical nature and polarity.
Observe any disruptions or precipitate formations during experiments to deduce stability conditions.
Introduction to Concentration
Concentration in Solution Chemistry:
- Concentration: A measure of the amount of solute present in a solution.
- Solute: The substance that is dissolved.
- Solvent: The medium that dissolves the solute.
- Solution: A homogeneous mixture of solute and solvent.
Definitions Recap
- Concentration: Amount of solute per unit of solvent or solution.
- Solute: The substance dissolved.
- Solvent: The medium dissolving the solute.
- Solution: Combination of solute and solvent.
Importance of concentration:
- Vital in:
- Medicine for precise dosages.
- Cooking for balanced taste.
- Chemical formulations.
Types of Concentration Measurements
Molarity (M):
- Molarity represents moles of solute per litre of solution.
- Formula: where = moles of solute, = volume in litres.
Worked Example for Molarity
Calculate the molarity for 40 g of NaCl in 500 mL of solution.
- Step 1: Determine moles of NaCl.
- Molar mass = 58.44 g/mol.
- Moles = .
- Step 2: Convert volume to litres.
- 500 mL = 0.5 L.
- Step 3: Compute Molarity
Key Tip: Ensure units are converted correctly for accurate results.
Common Errors in Molarity Calculations
- Forgetting to convert from millilitres to litres.
- Incorrect calculation of molar mass.
Mass Percent:
- Ratio of solute mass to solution mass, multiplied by 100.
Practice Problem for Mass Percent
Dissolve 20 g of sugar in 80 g of water. Calculate the mass percent.
- Step 1: Total mass of the solution:
- 20 g sugar + 80 g water = 100 g.
- Step 2: Calculate mass percent:
Solubility
Definition and Importance
Solubility: The maximum quantity of solute that can be dissolved under specific conditions.
- Industry Relevance: Crucial in processes and formulations.
- Everyday Use:
- Cooking: Sugar's influence on flavour and texture.
- Cleaning: Detergents dissolving stains efficiently.
Factors Affecting Solubility
Temperature:
- Solids dissolve better when heated, gases less so.
Pressure:
- Greater pressure boosts gas solubility, as seen in carbonated beverages.
- Henry's Law: Gas solubility increases proportionate to pressure.
Chemical Nature:
- "Like dissolves like": Polar substances dissolve in polar mediums, non-polar in non-polar.
- Example: Sugar in water (polar), oil in benzene (non-polar).
Remember, "like dissolves like" is pivotal for practical applications!
Factors vs. Solubility Table
| Factor | Effect on Solubility |
|---|---|
| Temperature | Solids: High solubility with heat; Gases: Low solubility with heat |
| Pressure | Minimal effect on solids/liquids; Gases: Solubility rises with pressure |
| Chemical Nature | Adheres to 'like dissolves like' principle |
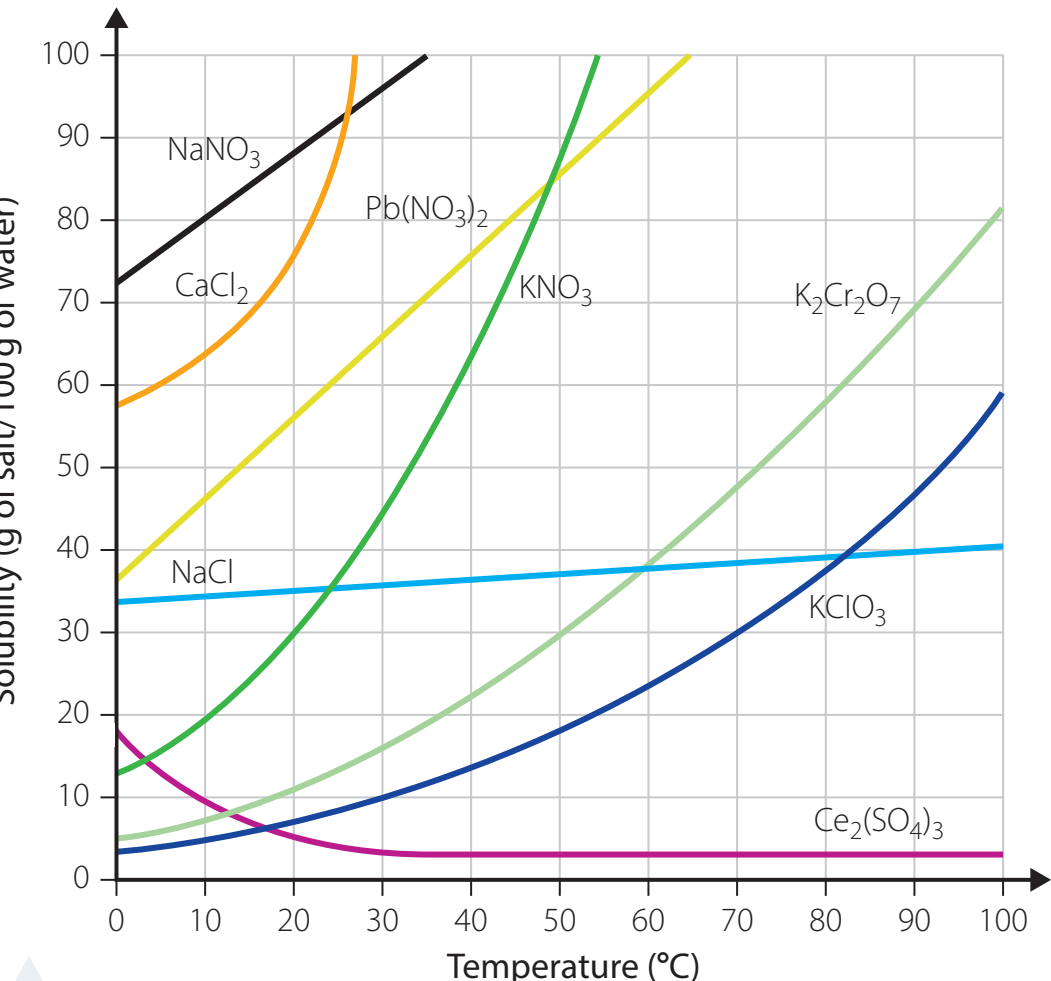
Solubility Curves
- Visual Aid: Graphs illustrating solubility changes with temperature.
- Reading Curves:
- Identify temperature, plot intersection, and determine solubility.
- Uses: Predict substance precipitation likelihood.
Practical Application Examples
- Baking: Sugar solubility affects flavour consistency.
- Medicine: Solubility impacts drug effectiveness and absorption.
Practical Investigations
Importance
- Hands-On Experiments: Connect theoretical concepts with practical experience.
- Common Observations: Observing solution chemistry in daily activities like dissolving sugar in lemonade.
Conducting Experiments
Materials and Equipment
- Essential tools include beakers, pipettes, volumetric flasks, solutes, and solvents.
- Techniques prioritise avoiding parallax errors and using calibrated tools.
Key Experiments
Solubility Curves
- Thoroughly explore temperature effects on solubility by controlling experimental conditions.
Ionic Compound Solubility
- Study solubility across different solvents, noting any residue formation.
Gas Solubility
- Demonstrate the effect of temperature on gas release through carbonated water.

Reflection and Analysis
- Utilise comprehensive data tables for clear data recording.
- Apply graphing techniques for visualising solubility variations.
Safety Considerations
- Always prioritise safety by following protocols, wearing goggles and gloves, and being prepared for spills.
Solubility
Overview
- Solubility is vital in numerous fields including pharmaceuticals and environmental science.
Problem Solving in Solution Chemistry
Strategies
- Emphasise unit analysis to maintain consistent measurement units.
Definitions - Molarity: Molarity (c) refers to the concentration measured in moles per litre (mol/L).
Calculations
Example
-
Basic Molarity Calculation:
- Convert and apply the formula:
-
Advanced Calculations: Incorporate mass/volume percent conversions for practical relevance.
500K+ Students Use These Powerful Tools to Master Introduction to Solutions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
442 flashcards
Flashcards on Introduction to Solutions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards40 quizzes
Quizzes on Introduction to Solutions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes7 questions
Exam questions on Introduction to Solutions
Boost your confidence with real exam questions.
Try Chemistry Questions2 exams created
Exam Builder on Introduction to Solutions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Introduction to Solutions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Introduction to Solutions you should explore
Discover More Revision Notes Related to Introduction to Solutions to Deepen Your Understanding and Improve Your Mastery
