Photo AI
Last Updated Sep 24, 2025
Electron-Dot Structures Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Electron-Dot Structures quickly and effectively.
451+ students studying
Electron-Dot Structures
Introduction
Lewis structures are fundamental for understanding chemical bonding and predicting molecular geometry. Why are they crucial for predictions? Comprehending these notations allows for an accurate depiction of molecular interactions and behaviour.
- Valence Electrons: Electrons present in the outermost electron shell of an atom. These electrons play a critical role in an atom's reactivity and bonding capabilities.
- Electron-Dot Structures: Known as Lewis dot diagrams, these notations visually represent an atom's valence electrons, illustrating their role in bond formation through sharing or transfer.
Valence electrons are vital in chemical reactions, as they dictate the way atoms interact.
Definition of Electron-Dot Structures
Electron-Dot Structures: Also referred to as Lewis dot diagrams, these visualise an atom's valence electrons. They provide a framework for understanding how electron sharing or transfer occurs during bonding.
Purpose of Electron-Dot Structures
- Illustrate electron sharing or transfer.
- Predict molecular interactions and bonding patterns.
- Facilitate understanding of chemical processes.
Gilbert N. Lewis's Contributions
- Gilbert N. Lewis devised electron-dot structures, which are fundamental to contemporary chemistry.
- His contributions enhance our knowledge of atomic bonding and molecular behaviour.
- Did you know? Lewis's contributions are foundational in explaining molecular dynamics.
Understanding Electron-Dot Diagrams
- Electron-dot diagrams: Dots surrounding an element's symbol indicate its valence electrons.
- Follow Hund's Rule: distribute electrons singly in orbitals before pairing them.
Hund's Rule: Electrons fill empty orbitals singly before pairing occurs.
Predicting Reactivity Using Diagrams
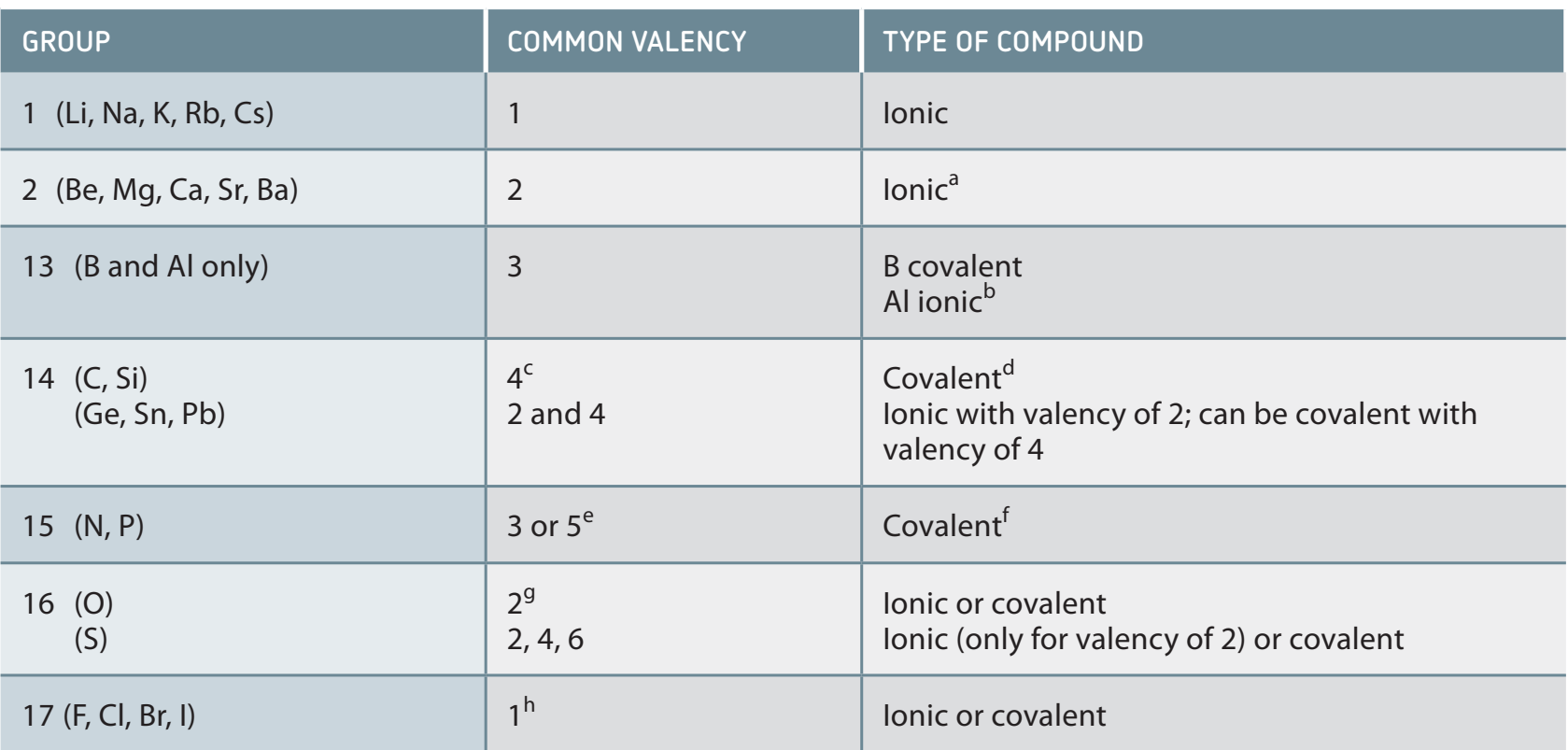
- Example: Chlorine (Cl) often gains an electron to stabilise, resulting in sodium chloride.
Visual Aids and Examples
-
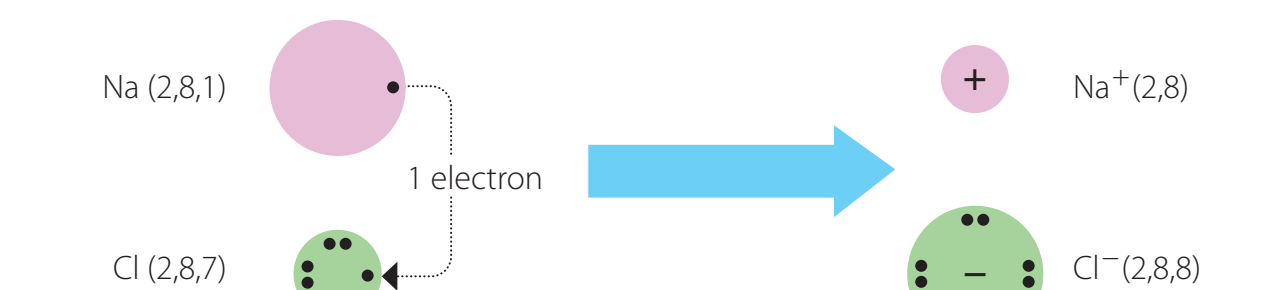
Oxygen's electron configuration () reveals 6 valence electrons; Carbon (Group 14) has 4.
-
Oxygen Atom Example: Dots indicate valence electrons, essential in bond formation.

- Water (H₂O): Each hydrogen atom shares an electron with oxygen.
Introduction to Ionic Compounds
Ionic Compounds: Created by electron transfer, leading to ion formation.
- Cations: Positively charged ions formed through electron loss.
- Anions: Negatively charged ions formed through electron gain.
Key Terms:
- Cations: Positive ions.
- Anions: Negative ions.
Explanation of Ionic Bonds
Ionic Bonds: The robust electrostatic attraction between cations and anions.
- Ionic bonds are strengthened in comparison to covalent bonds. They ensure stability by enabling a noble gas configuration, akin to magnets with powerful pulls between opposing charges.
Understanding Bond Strength: Ionic bonds are crucial for maintaining compound stability due to their strong attractions.
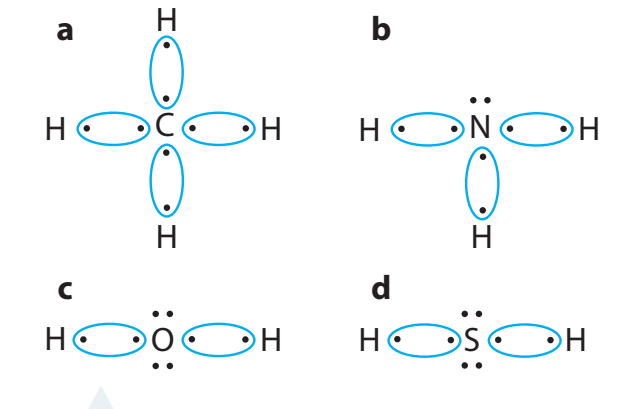
Example: Sodium Chloride (NaCl)
- Sodium (Na) loses one electron, becoming Na⁺.
- Chlorine (Cl) gains one electron, becoming Cl⁻.
Introduction to Covalent Bonds
Covalent bonds form when atoms share electrons to achieve stability.
- Bond Pairs: Electrons shared between atoms.
- Lone Pairs: Non-bonding electrons, affecting molecular shape and reactivity.
- Bond Pairs: Electrons shared to establish bonds between atoms.
- Lone Pairs: Non-bonding electrons influencing molecular shape and reactivity.
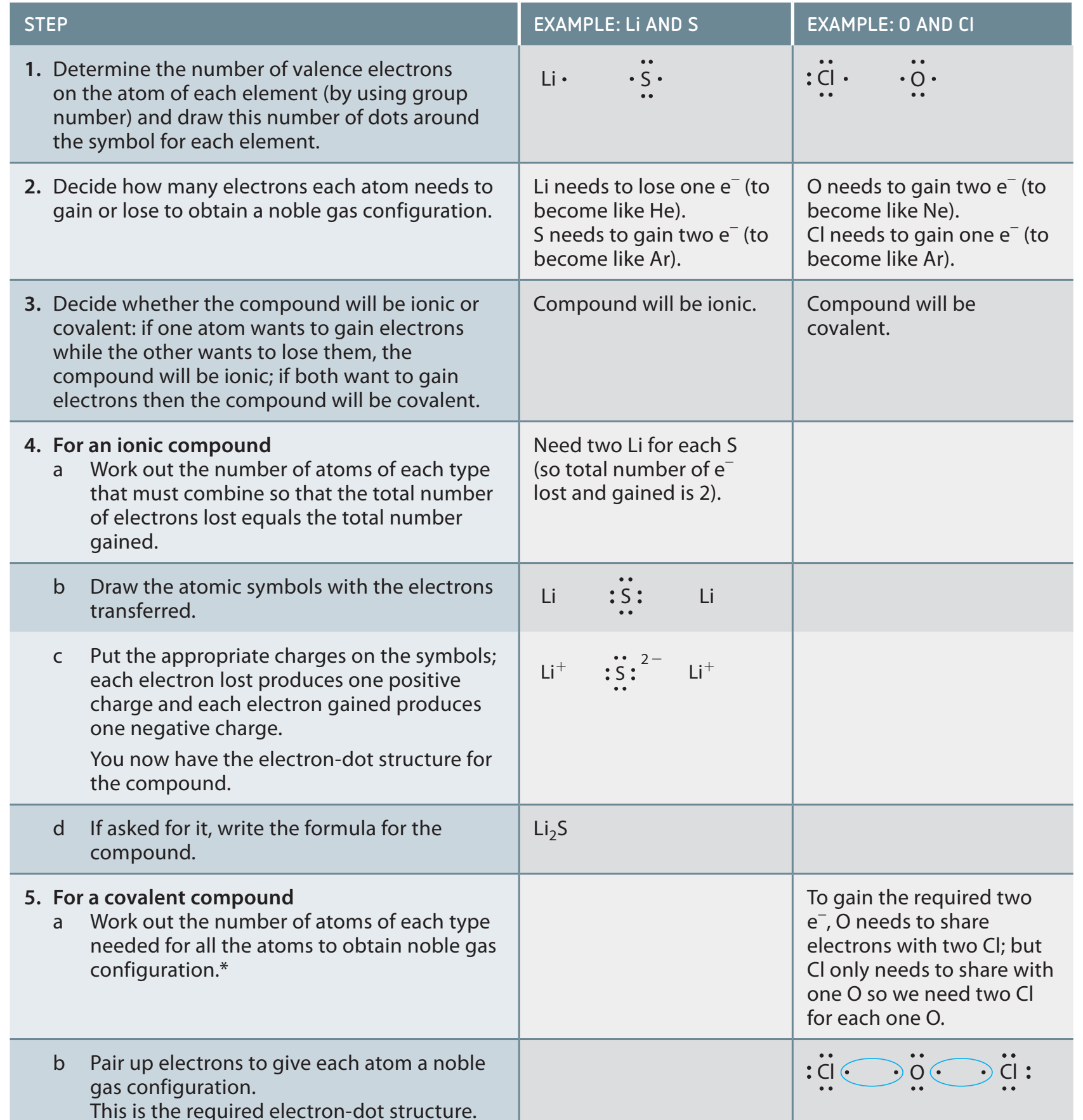
Step-by-Step Guide to Drawing Lewis Structures
- Determine Valence Electrons
- Atom Arrangement
- Sketching Initial Bonds
- Electron Distribution
- Using Multiple Bonds

Common Mistakes and Tips for Lewis Structures
1. Introduction to Common Mistakes
- Miscounting Valence Electrons
- Incorrect Electron Placements
- Overlooking Charge Balance
- Misuse of Formal Charges
2. Miscounting Valence Electrons
Remember, valence electrons dictate atomic bonding patterns. Miscounts can lead to unstable structures.
- Why the Mistake Happens: Misinterpretation of group numbers in the periodic table.

3. Incorrect Electron Placements
- Example: Oxygen requires double bonds to satisfy the octet rule.
4. Overlooking Charge Balance
Always check ionic compounds for charge balance using the mnemonic "Zero Sum for Stability."
5. Verification with Formal Charges
Importance: Formal charges are essential for confirming structural stability. Calculating them can reveal and correct errors.
Practice Exercises with Solutions
Exercise 1: Hydrogen (H₂) Solution: Two hydrogen atoms each with one valence electron share their electrons to form a single bond (H-H).
Exercise 2: Oxygen (O₂) Solution: Two oxygen atoms (each with 6 valence electrons) form a double bond with two lone pairs on each oxygen atom.
Exercise 3: Ammonia (NH₃) Solution: Nitrogen (5 valence electrons) forms three single bonds with hydrogen atoms and has one lone pair.
Exercise 4: Sulfur Dioxide (SO₂) Solution: Central sulfur atom (6 valence electrons) forms one single bond and one double bond with oxygen atoms, with one lone pair on sulfur.
Exercise 5: Carbon Dioxide (CO₂) Solution: Central carbon atom (4 valence electrons) forms double bonds with each oxygen atom.
Exercise 6: Sulfate Ion (SO₄²⁻) Solution: Central sulfur atom (6 valence electrons) forms single bonds with each oxygen atom, with each oxygen carrying a negative charge. The overall charge is 2-.
500K+ Students Use These Powerful Tools to Master Electron-Dot Structures For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
374 flashcards
Flashcards on Electron-Dot Structures
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards36 quizzes
Quizzes on Electron-Dot Structures
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes11 questions
Exam questions on Electron-Dot Structures
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Electron-Dot Structures
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Electron-Dot Structures
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Electron-Dot Structures you should explore
Discover More Revision Notes Related to Electron-Dot Structures to Deepen Your Understanding and Improve Your Mastery
