Photo AI
Last Updated Sep 24, 2025
Noble Gas Configurations Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Noble Gas Configurations quickly and effectively.
296+ students studying
Noble Gas Configurations
Definition of Noble Gases
Noble Gases: Elements located in Group 18 of the periodic table, known for their low chemical reactivity due to a stable electronic configuration.
Contextual Insight: Noble gases, including helium and neon, exhibit minimal reactivity as their electrons are arranged in complete shells. Helium is lighter than air and non-flammable, making it suitable for use in balloons.
Overview and Characteristics of Noble Gases
- Members: Helium, Neon, Argon, Krypton, Xenon, Radon.
- Properties:
- Non-reactive at room temperature, indicating they resist forming compounds.
- Colorless and odourless gases.
These properties make them ideal for various technological applications, including lighting and cryogenics.
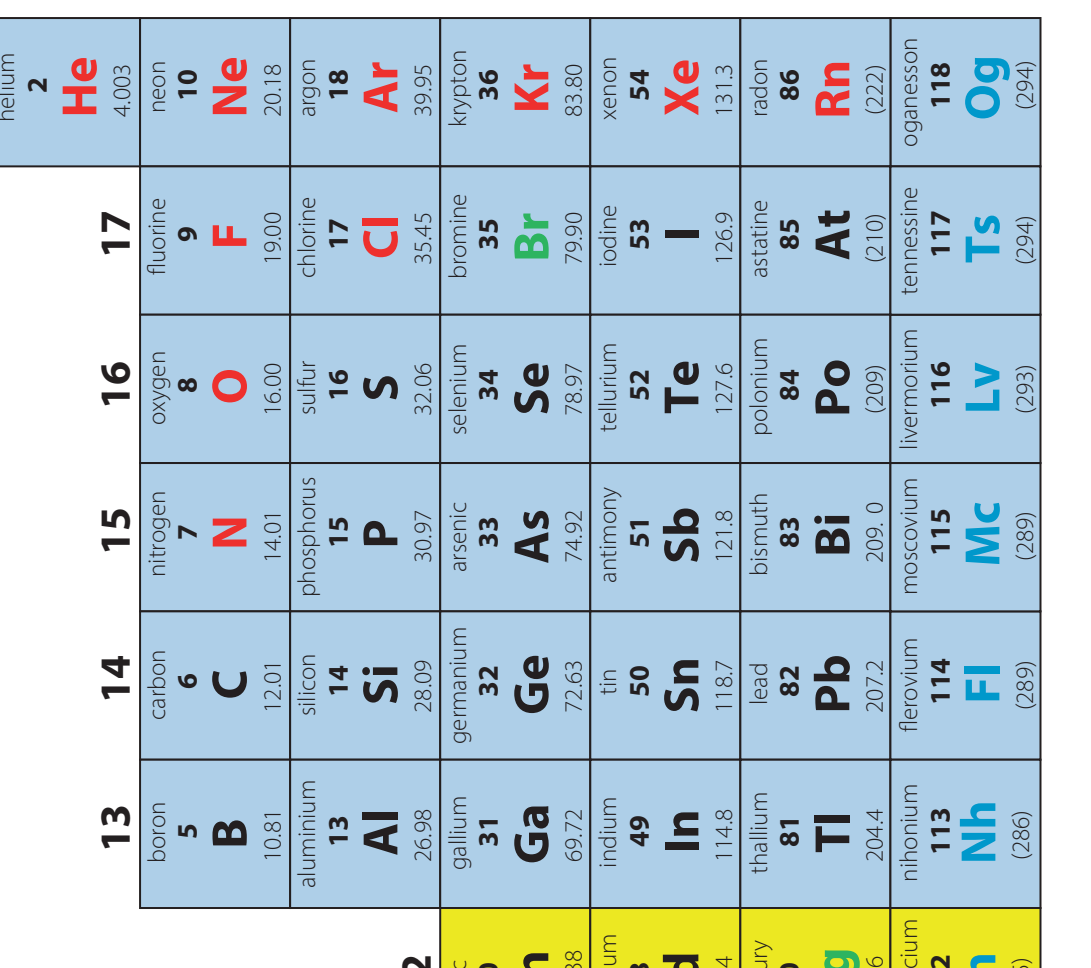
Noble Gas Electron Configurations
Introduction
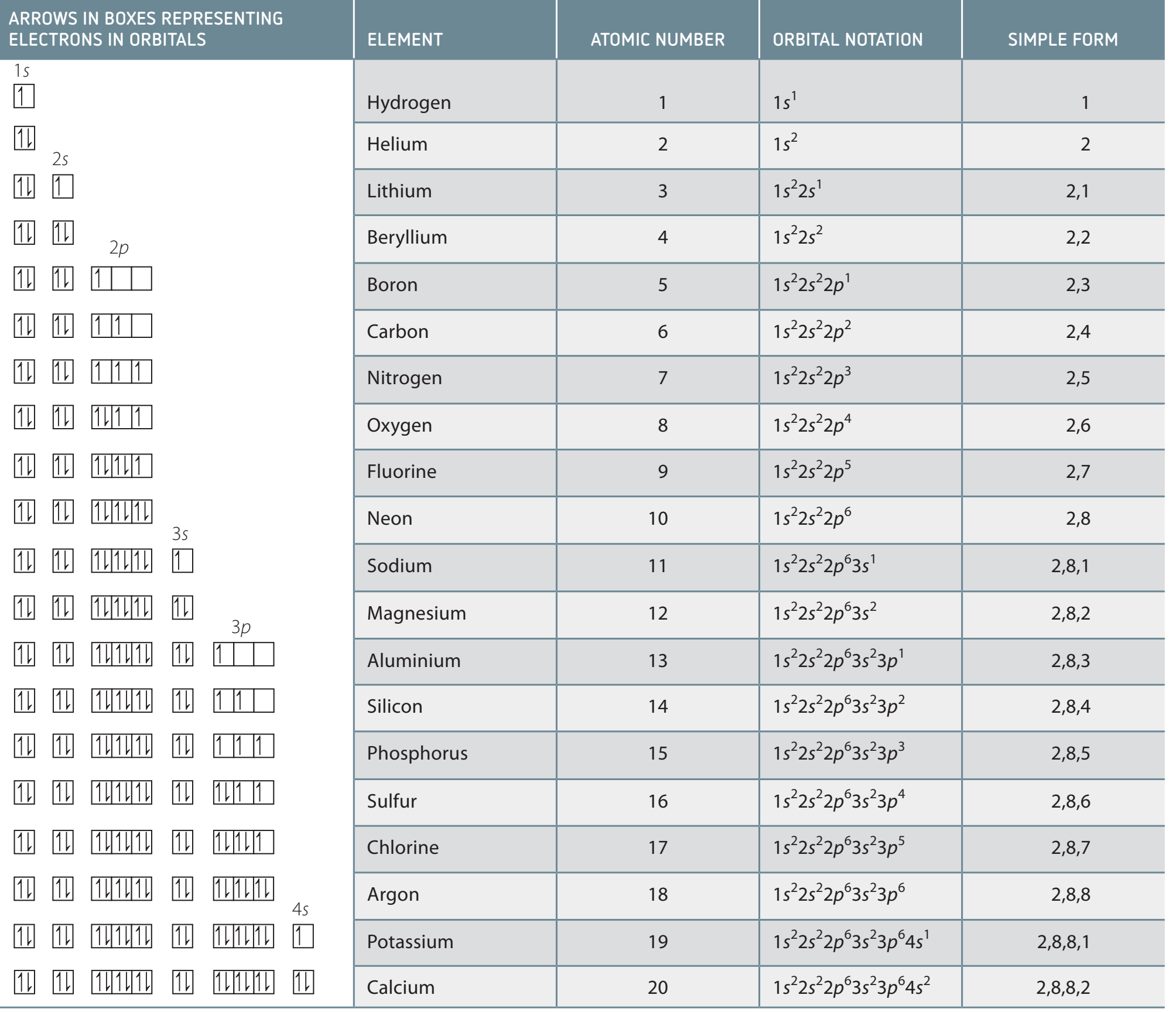
- Electron configurations: The arrangement of electrons in the shells of an atom, crucial for predicting the chemical and physical properties of elements.
- Noble gas configurations: Models for atomic stability due to fully filled outer shells. These stable configurations contribute to the low reactivity of an element.
Full Valence Shells and Stability
- Electron Configuration: Noble gases possess a full valence shell, which provides exceptional stability and a reluctance to engage in chemical reactions.
Electron Configurations:
- Helium:
- Neon:
- Argon:
- Krypton:
- Xenon:
- Radon:
Diagrammatic Representation
-
Atomic Structure Diagrams: Shell models display noble gases with fully occupied valence shells.

-
Comparison Diagrams: Highlighting shell completeness differences underscores the stable nature of noble gases.

Noble gases versus other elements: Stability is achieved with complete electron shells.
The Octet Rule
Definition: The octet rule asserts that atoms are most stable when holding eight electrons in their outermost shell, akin to noble gases.
- Significance: It is instrumental in predicting chemical bonding and behaviour.
The octet rule serves as a basis for understanding chemical stability and reactivity.
Influence on Chemical Bond Formation
-
Ionic Bonds: Electrons are transferred between atoms to form stable ionic structures, emulating noble gases.
-
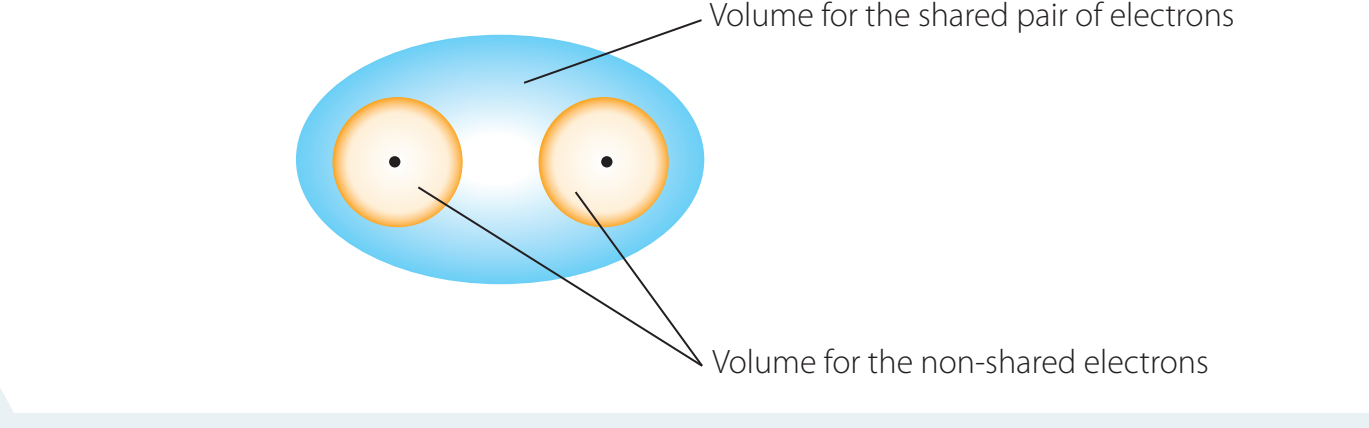
Covalent Bonds: Atoms share electrons to complete their valence shells, achieving stability.
Understanding Ionic Stability
-
Noble Gas Configuration: This refers to a completely filled outer electron shell. Atoms attain stability by acquiring this configuration via ion formation.
-
Ions: Charged particles produced by electron gain or loss.
- Cations: Positively charged ions resulting from electron loss.
- Anions: Negatively charged ions formed by electron gain.
- Ions: Particles with a net electric charge due to electron transfer.
- Cations: Positively charged by losing electrons.
- Anions: Negatively charged by gaining electrons.
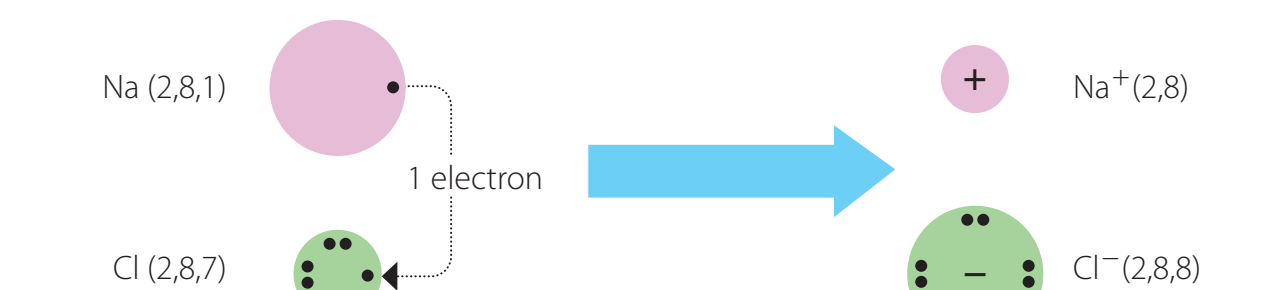
Example: Sodium Chloride Formation
- Na loses an electron, forming Na⁺.
- Cl gains this electron, resulting in Cl⁻.
- Na⁺ and Cl⁻ are connected through electrostatic attraction forming NaCl.
Introduction to Covalent Bonding
-
Covalent bonding: Electron sharing among nonmetals to reach configurations akin to noble gases.
-
Examples of Covalent Bonding:
Methane (CH₄)
- Carbon shares electrons with four hydrogen atoms, acquiring stability.
Water (H₂O)
- Electrons are shared with hydrogen, forming polar covalent bonds.
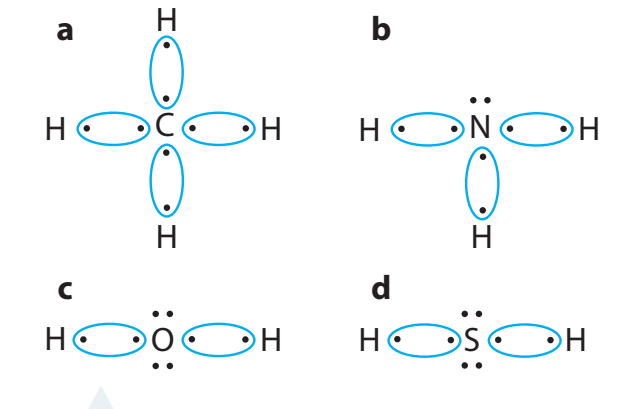
The essence of covalent bonds is to achieve stability comparable to noble gases, facilitating molecular integrity.
Practice Problems
Ionic Bond Examples
- Sodium Chloride (NaCl)
- Sodium (Na):
- Action: Sodium loses one electron.
- Result: Forms Na⁺ ion, a positive ion.
- Chlorine (Cl):
- Action: Chlorine gains one electron.
- Result: Becomes Cl⁻ ion, a negative ion.
- Sodium (Na):
Covalent Bond Examples
-
Methane (CH₄):
- Solution: Carbon (4 valence electrons) shares one electron with each of the four hydrogen atoms (1 valence electron each). This sharing gives carbon 8 electrons in its outer shell (like neon) and each hydrogen 2 electrons (like helium).
-
Water (H₂O):
- Solution: Oxygen (6 valence electrons) shares one electron with each of two hydrogen atoms. This gives oxygen 8 electrons in its outer shell and each hydrogen 2 electrons, achieving noble gas configurations for all atoms.
Key Understanding: Noble gases are widely used because of their non-reactive properties, making them safe and reliable for various applications.
500K+ Students Use These Powerful Tools to Master Noble Gas Configurations For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
374 flashcards
Flashcards on Noble Gas Configurations
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards36 quizzes
Quizzes on Noble Gas Configurations
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes11 questions
Exam questions on Noble Gas Configurations
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Noble Gas Configurations
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Noble Gas Configurations
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Noble Gas Configurations you should explore
Discover More Revision Notes Related to Noble Gas Configurations to Deepen Your Understanding and Improve Your Mastery
