Photo AI
Last Updated Sep 24, 2025
Ionic Bonding Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Ionic Bonding quickly and effectively.
394+ students studying
Ionic Bonding
Overview
- Chemical bonding is pivotal in determining the behaviour and interaction of substances. Understanding bonding explains various phenomena, such as the formation of common salts and unique properties of water.
A comprehensive understanding of chemical bonding elucidates many phenomena encountered in everyday life, such as salt formation and water's behaviour.
Key Definitions
-
Chemical Bond: An attractive force that holds atoms together in molecules or compounds.
chatImportantA chemical bond is the attractive force maintaining atoms within molecules or compounds.
-
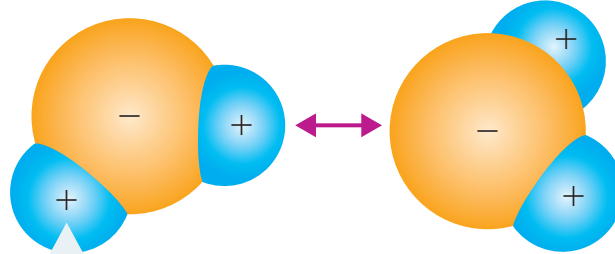
Ionic Bond: A type of chemical bond characterised by the transfer of electrons from one atom to another, resulting in ion formation.
Role of Electrons
- Electrons: Crucial for bond formation. They are transferred, shared, or delocalised between atoms.
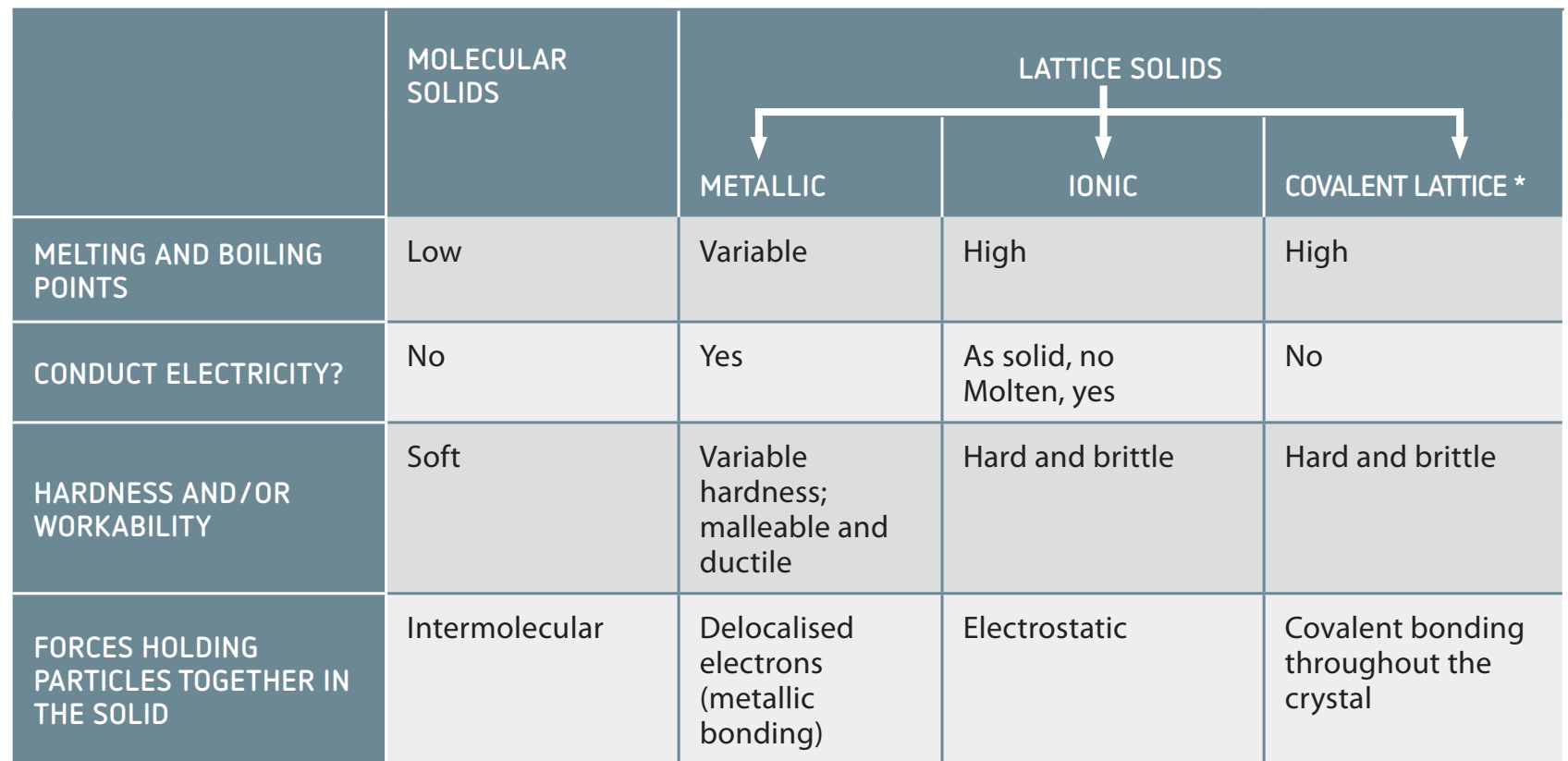
Types of Chemical Bonds
- Ionic Bonds: Form through electron transfer, typically between metals and non-metals; e.g., Sodium chloride creates crystal structures.
- Covalent Bonds: Involve electron sharing between non-metals; e.g., Water .
- Metallic Bonds: Feature delocalised electrons in a 'sea of electrons' surrounding positive metal ions; e.g., Iron .
| Type of Bond | Electron Activity | Typical Elements Involved |
|---|---|---|
| Ionic | Electron Transfer | Metal with Non-metal |
| Covalent | Electron Sharing | Non-metal with Non-metal |
| Metallic | Electron Delocalisation | Metal with Metal |
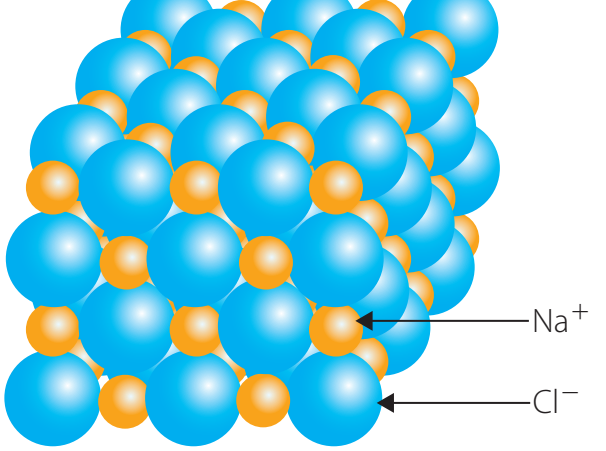
Characteristics of Ionic Bonds
- Ionic bonds form crystalline structures with high melting points.
- Compounds are highly soluble and conduct electricity well when dissolved, due to dissociated ions.
- Strong electrostatic forces lead to bond stability.
Ionic bonds result in compounds like , creating solid crystals and providing stability.
Electronegativity
-
Electronegativity: The ability of an atom to attract and hold electrons.
infoNoteElectronegativity: Determines bond character based on electron attraction strength.
-
Pauling Scale: Assigns values to describe elemental behaviour in bonds—high values, like 3.98 for Fluorine, indicate a strong attraction to electrons.
-
Electronegativity Differences:
- > 1.5: Indicates an ionic bond, e.g., sodium and chlorine.
- < 1.5: Suggests a covalent bond, e.g., hydrogen and oxygen.
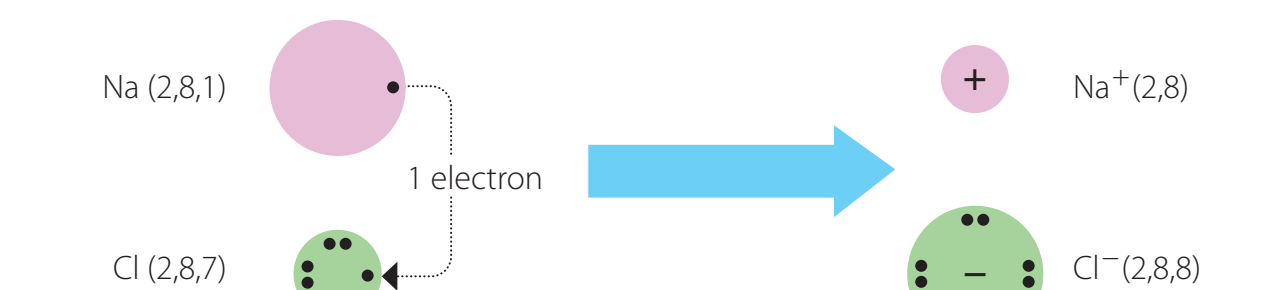
Formation of Ionic Bonds
- Ionic bonds form through electron transfer between metals and non-metals:
- Cations (+): Positively charged ions formed when metals lose electrons.
- Anions (-): Negatively charged ions formed when non-metals gain electrons.
Ions: Charged particles formed when atoms gain or lose electrons.

- Example: In sodium chloride :
- Sodium atom transfers an electron to chlorine, forming a sodium cation and a chloride anion.
- Strong electrostatic forces, or lattice structure, bind these ions, resulting in a stable compound.
Properties of Ionic Compounds
High Melting and Boiling Points
- Ionic compounds have high melting and boiling points due to strong electrostatic attractions between ions.

Lattice Energy's Role: Stronger lattice energy increases the energy needed to disrupt bonds, raising melting and boiling points.
Electrical Conductivity
- Solid State: Fixed ions cause no conductivity.
- Molten/Aqueous States: Free-moving ions enable conductivity.
State of Matter and Conductivity:
- Solid State: Non-conductive.
- Molten/Aqueous States: Conductive due to free-moving ions.
Solubility in Water
- Ionic compounds dissolve in water through interactions with polar water molecules.
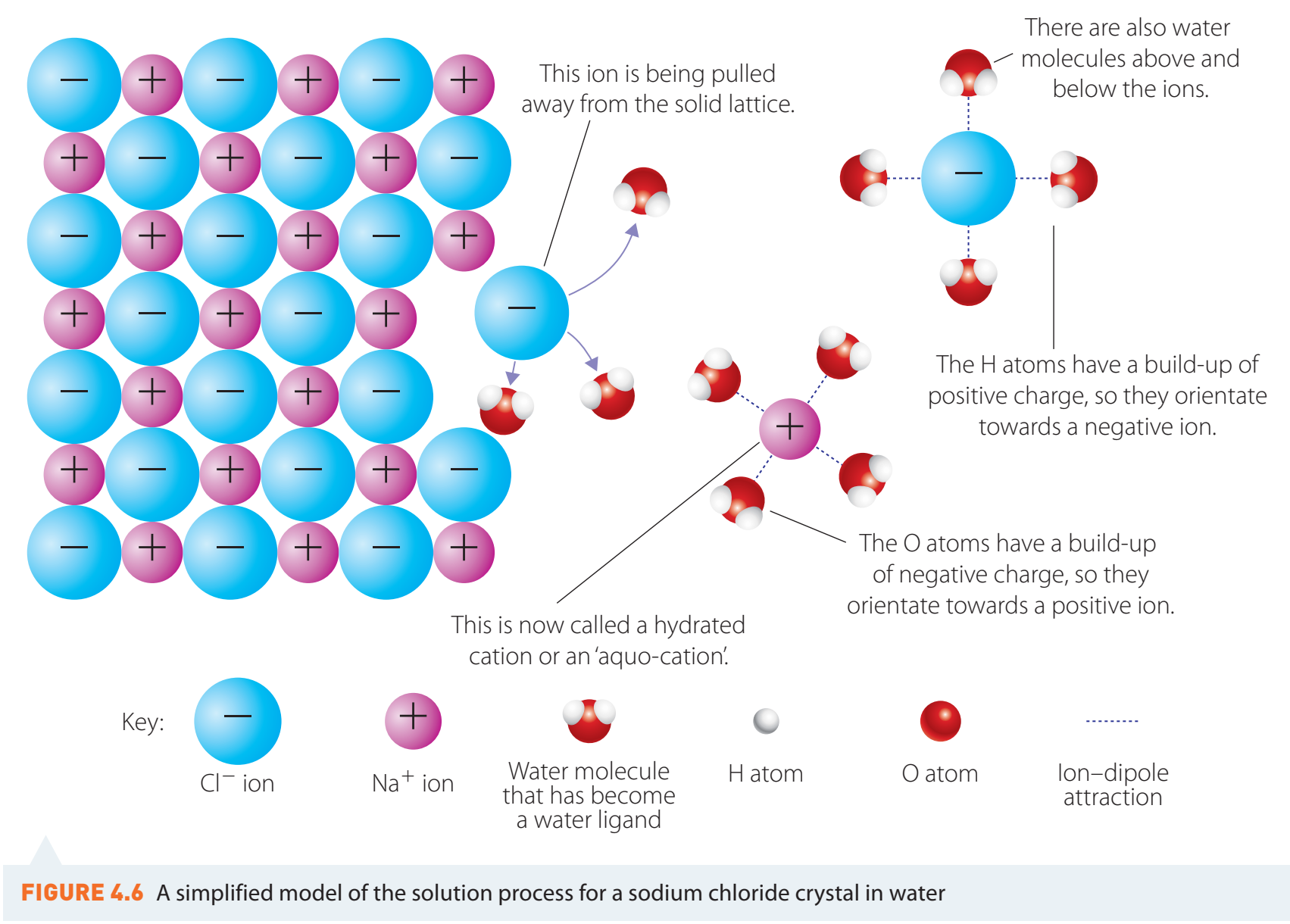
Hydration: Water molecules surround and interact with ions, aiding solubility.
Brittleness of Ionic Compounds
- Ionic compounds are brittle. Applied force aligns like-charged ions, causing repulsion and cleavage.
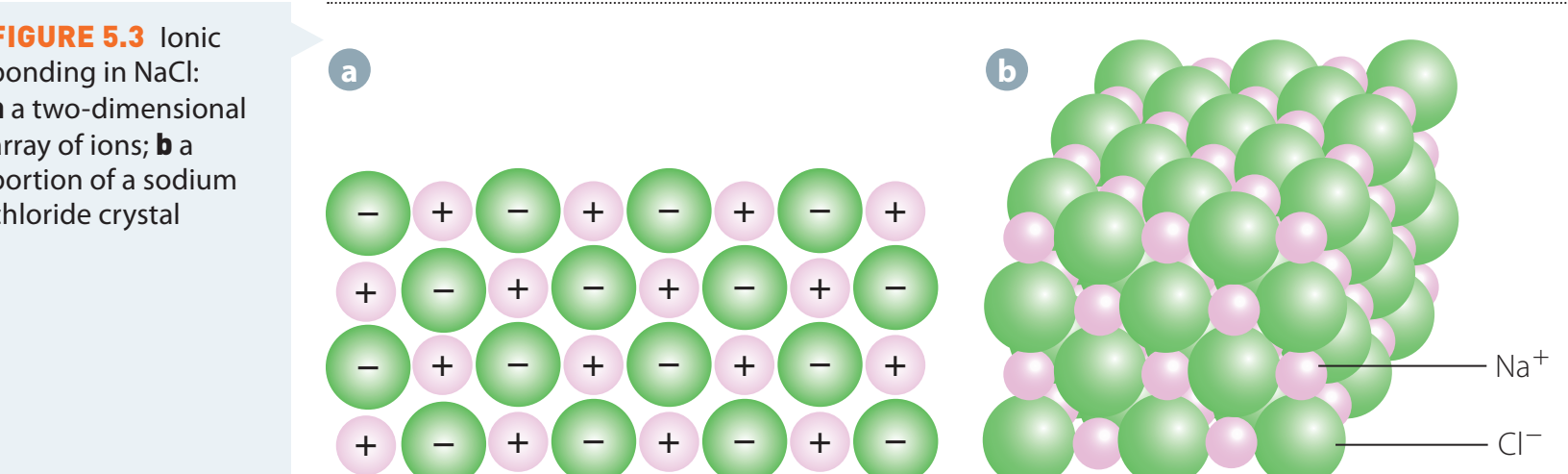
Ionic Networks and Crystal Lattices
Ionic Networks: Structures of ions arranged in stable patterns.
Ionic Networks: Contribute to stability and compound characteristics.
- Crystal Lattice: A repeating three-dimensional pattern ensuring stable ion alignment.
- Lattice Energy: Refers to the energy involved in forming or breaking ionic solids.
Influence of Electronegativity and Ionic Character
- Electronegativity Difference (ΔEN): Determines bond ionic character.
- Thresholds:
- ΔEN > 1.7: Indicates an ionic bond.
- ΔEN < 1.7: Often implies a covalent bond.

Calculating Percentage Ionic Character
- Formula:
- This estimates ionic character percentage based on ΔEN.
Worked Example: Formation of Sodium Chloride
Let's examine how sodium chloride forms through ionic bonding:
-
Starting Configuration:
- Sodium (Na): Electron configuration (1 valence electron)
- Chlorine (Cl): Electron configuration (7 valence electrons)
-
Electron Transfer:
- Sodium loses its single valence electron to form (more stable noble gas configuration)
- Chlorine gains this electron to form (achieving a full octet)
-
Bond Formation:
- The oppositely charged ions attract each other through electrostatic forces
- This attraction forms the ionic bond in NaCl
-
Verification:
- The electronegativity difference between Na (0.9) and Cl (3.0) is 2.1
- Since 2.1 > 1.7, this confirms the bond is predominantly ionic
This electron transfer creates a stable compound where both atoms achieve noble gas configurations, demonstrating how ionic bonds form through the complete transfer of electrons from metals to non-metals.
Real-World Connection
Ionic bonds are evident in everyday life, such as in table salt , contributing to health and nutrition.
500K+ Students Use These Powerful Tools to Master Ionic Bonding For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
374 flashcards
Flashcards on Ionic Bonding
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards36 quizzes
Quizzes on Ionic Bonding
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes11 questions
Exam questions on Ionic Bonding
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Ionic Bonding
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Ionic Bonding
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Ionic Bonding you should explore
Discover More Revision Notes Related to Ionic Bonding to Deepen Your Understanding and Improve Your Mastery
