Photo AI
Last Updated Sep 24, 2025
Relative Formula Mass Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Relative Formula Mass quickly and effectively.
334+ students studying
Relative Formula Mass
Introduction
Understanding the Relative Formula Mass (RFM) is essential in chemistry. It significantly influences quantitative analysis, such as balancing chemical equations and preparing accurate solutions in laboratory settings. For instance, RFM helps ensure the correct ratios of reactants and products during experiments.
Overview
- Purpose:
- Comprehend mass relationships in compounds.
- Fundamental to stoichiometry.
- Crucial component in chemical reactions.
- Role in chemical reactions as a critical factor.
RFM is vital for comparing compound weights and calculating quantities of reactants and products in equations.
Concept of Relative Formula Mass
- Relative Formula Mass (RFM): The sum of the relative atomic masses of all atoms in a formula unit. It is expressed as a unitless number.
Example: In NaCl, the RFM is determined by adding the atomic mass of Sodium (Na) to that of Chlorine (Cl), underscoring its important role in accurately measuring chemical quantities.
Distinction between Related Terms
- Molecular Mass vs. Formula Mass:
- Molecular Mass: Applicable to covalent compounds.
- Formula Mass: Applicable to ionic compounds.

When solving problems, apply formula mass for ionic compounds and molecular mass for covalent compounds. Recognising this distinction aids in selecting the correct method of calculation.
RFM Representation and Calculation
Step-by-Step Calculation
Step 1: Identify Elements
- Obtain the Chemical Formula: Identify each element along with its corresponding quantity.
- Example: Enumerate each element with the total number of atoms.
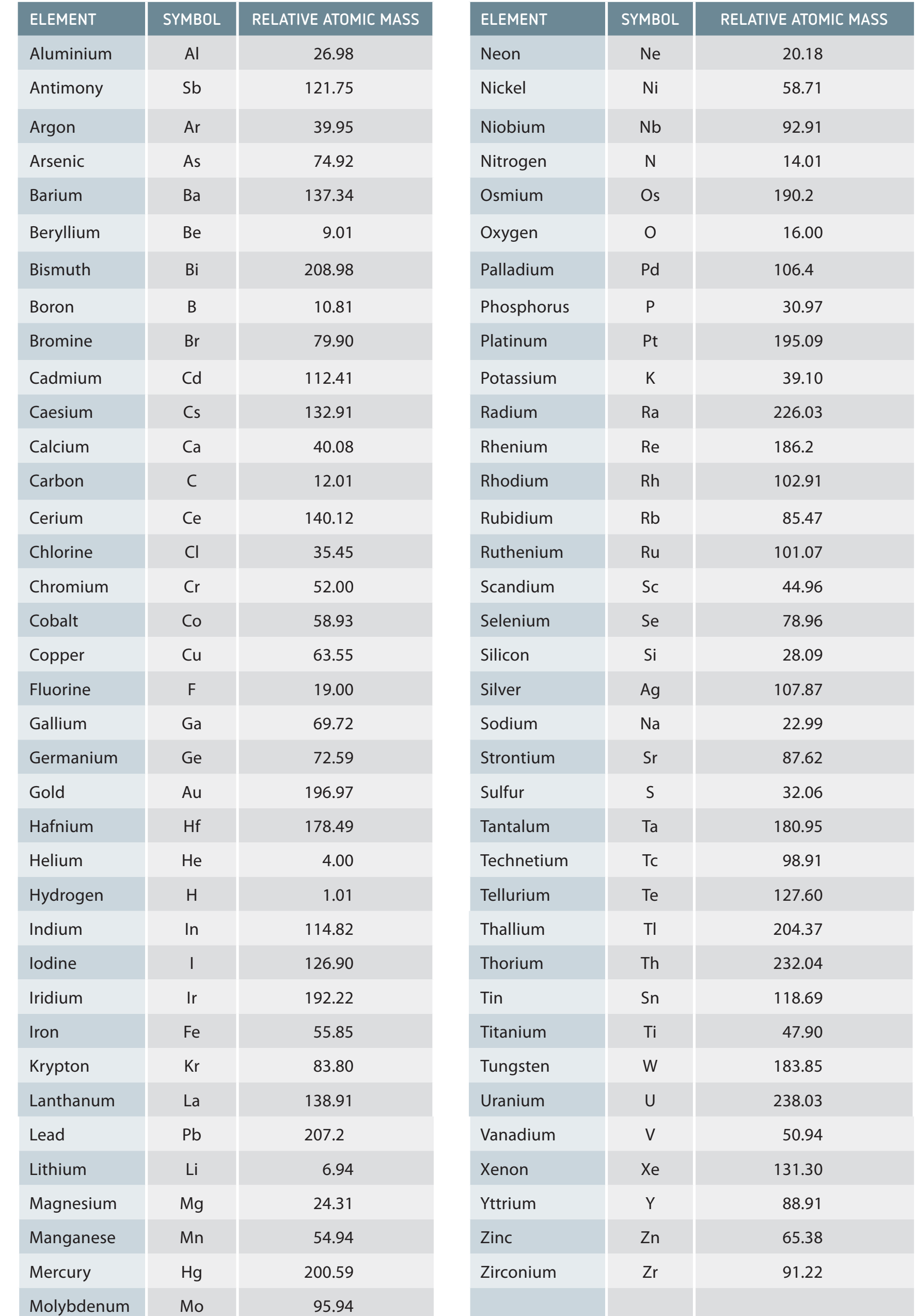
Step 2: Find Atomic Masses
- Use the Periodic Table: Locate and interpret atomic masses.
- Read atomic masses displayed diagonally beneath each element's symbol.

Utilise the periodic table's diagonal arrangement for precise atomic mass identification.
Step 3: Multiply and Sum
- Multiply the atomic mass of each element by its number of atoms.
- Sum these results to derive the RFM.

Worked Examples
- Sodium Chloride (NaCl):
- Sodium (Na): Relative mass = 23
- Chlorine (Cl): Relative mass = 35.5
- RFM of NaCl:
- Calcium Fluoride (CaF_2):
- Calcium (Ca): Relative mass = 40
- Fluorine (F): Relative mass = 19
- RFM of CaF:
-
Example: Calculating RFM of H₂SO₄
- Identify Elements: H: 2, S: 1, O: 4
- Atomic Masses: H: 1, S: 32, O: 16
- Calculations:
- Hydrogen:
- Sulphur:
- Oxygen:
- RFM Total:
-
Example: Calculating RFM of C₆H₁₂O₆
- Interpret Elements: C: 6, H: 12, O: 6
- Atomic Masses: C: 12, H: 1, O: 16
- Calculations:
- Carbon:
- Hydrogen:
- Oxygen:
- RFM:
Always double-check calculations and data for accuracy.
Applications in Stoichiometric Calculations
In this section, we explore stoichiometry's application in chemical calculations, focusing on Relative Formula Mass (RFM).
Relative Formula Mass (RFM): RFM is the total atomic masses of all atoms in a molecule. It facilitates conversions between moles and grams, crucial for precise quantification in reactions.
Stoichiometry in Practice
-
Conversion Process:
- Identify Given Quantities: Start with the given masses or moles.
- Convert Masses to Moles: Use RFM.
- Apply Mole Ratios: Ensure equations are balanced for accurate ratios.
- Convert Moles to Mass: Utilise RFM if a mass is required.
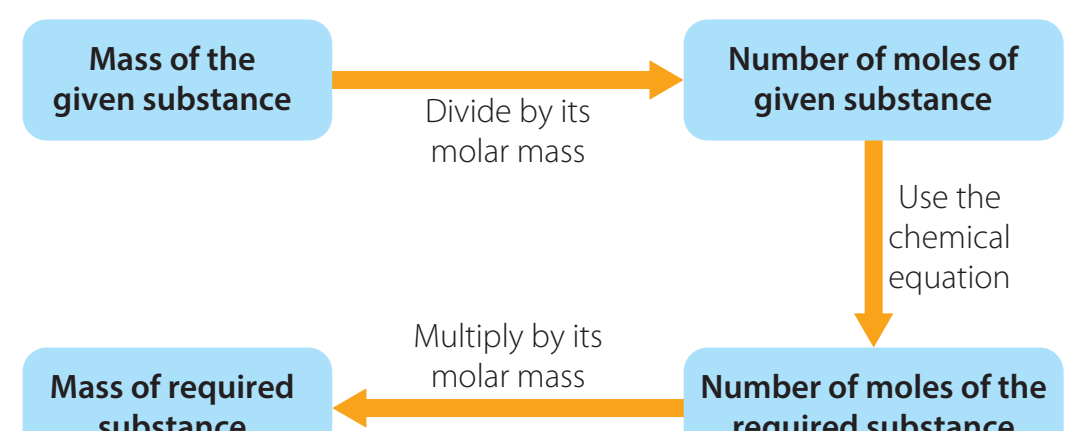
-
Balanced Equation Importance:
- Balances atoms: Vital for precision.
- Use callouts to remember balancing tips.
chatImportantAlways balance equations prior to calculation!
Common Misconceptions
- Misconceptions around RFM:
- Isotopic Variations: Isotopes generally do not affect basic RFM calculations.
- Confusing RFM with Actual Mass: Use correct formula masses in calculations.
- Corrective Strategies:
- Verify atomic masses with reliable periodic tables.
- Confirm and use appropriate compound structures.
Always remember, RFM is a unitless number. Utilise the atomic masses as listed in the periodic table for accurate calculations.
Common Challenges
- Mole Ratio Understanding & Balancing:
- Clarify explanations with visuals.
- Visual aids significantly enhance students' comprehension of these concepts.

Practice Question
-
Calculate the RFM of water (H₂O) using atomic masses from the periodic table.
Solution:
- Hydrogen (H): Relative mass = 1, Quantity = 2
- Oxygen (O): Relative mass = 16, Quantity = 1
- RFM of H₂O =
500K+ Students Use These Powerful Tools to Master Relative Formula Mass For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
234 flashcards
Flashcards on Relative Formula Mass
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Relative Formula Mass
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes21 questions
Exam questions on Relative Formula Mass
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Relative Formula Mass
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Relative Formula Mass
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Relative Formula Mass you should explore
Discover More Revision Notes Related to Relative Formula Mass to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Chemical Reactions and Stoichiometry
Chemical Reactions Quantitative Calculations
261+ studying
186KViews96%
114 rated
Chemical Reactions and Stoichiometry
Law of Conservation of Mass
239+ studying
188KViews