Photo AI
Last Updated Sep 24, 2025
Entropy and Gibbs Energy Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Entropy and Gibbs Energy quickly and effectively.
271+ students studying
Entropy and Gibbs Energy
Introduction to Thermodynamics
Key Concept: Thermodynamics explores energy transformations within physical and chemical systems, enhancing understanding of scientific principles and everyday phenomena.
Basic Concepts
- System: The section of the universe being studied.
- Surroundings: All external aspects interacting with the system.
- Universe: The total of the system and surroundings.
Significance of System Definitions: Precise system definition is essential for accurate energy process analysis. Incorrect definitions can result in errors, such as neglecting environmental heat exchange, which could distort energy balances.
Laws of Thermodynamics
-
First Law (Conservation of Energy): Energy cannot be created or destroyed, only converted from one form to another.
- Example: When a system performs work, like gas compression, its internal energy decreases.
-
Second Law: Entropy of an isolated system will never decrease over time.
- Example: Refrigerators facilitate heat transfer from colder to warmer areas, increasing total system entropy.
-
Third Law: At absolute zero, the entropy of a perfect crystal is zero, reflecting complete molecular order.
- Mathematical Clarity: .
Distinctions Between Energy Types
- Energy: The ability to perform work or generate heat.
- Thermodynamic Energy: Comprises particular energies like enthalpy (heat content) and Gibbs Free Energy (potential for spontaneity).
Energy Transformations in Practice: Conversions like kinetic to thermal energy in power generation illustrate significant thermodynamic applications.
Foundation for Further Study
- A grasp of entropy, enthalpy, and Gibbs Free Energy is vital for evaluating reaction spontaneity and deeper thermodynamic concepts.
- Lays groundwork for advanced studies including the significance of Gibbs Free Energy.
Visual Aid
Integrating these fundamental concepts enables students to approach complex topics like Gibbs Free Energy and its role in chemical reactions.
Entropy: A Measure of Disorder
Key Concepts and Definitions
- Entropy (S):
- Definition: A metric of a system's energy unavailable for useful work.
- Significance: Reflects energy spread within a system.
- Units: Measured in Joules per Kelvin (J/K), quantifying energy unavailability.
Entropy and Disorder
- Correlation with Disorder:
- Entropy Quantification: Evaluates how disorder and energy spread are measured within a system.
- Common Misconception: Highlights that entropy encompasses more than disorder, representing the extent of energy distribution.
Entropy Change During Phase Transitions
- Phase Transitions and Reactions:
- Transitions from solid to liquid to gas involve increased entropy:
- Solid to Liquid: Entropy rises as solid structures dissolve.
- Liquid to Gas: Entropy further increases as molecules disperse upon vaporisation.
- Entropy Change Formula (): Apply , emphasising formula application to reinforce understanding.
- Transitions from solid to liquid to gas involve increased entropy:
Examples of Entropy Changes
- Practical Examples:
- Melting Ice: Demonstrate the process with straightforward energy absorption leading to disorder rise and transition to liquid.
- Calculation Example: Follow a step-by-step process using for 1 mole of ice.
- Dissolving Salt in Water: Describe how entropy increases as particles spread, showcasing increased randomness.
- Melting Ice: Demonstrate the process with straightforward energy absorption leading to disorder rise and transition to liquid.
Visual Representation
- Diagrams and Models:
- Explicitly reference diagrams to assist application.

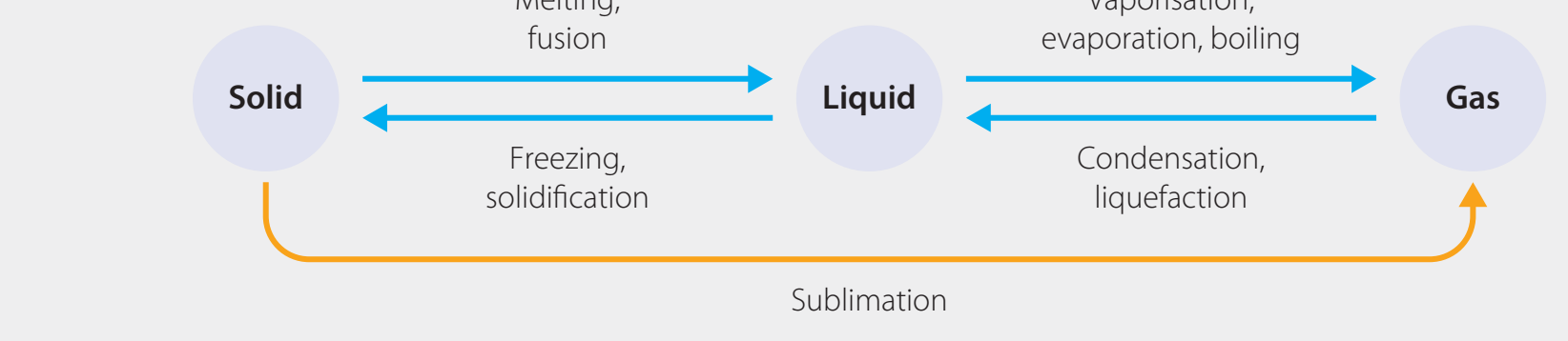
- Explicitly reference diagrams to assist application.
Important Concepts and Highlights
- Key Points to Highlight:
- Temperature's Effect on Entropy: Increasing temperatures typically elevate entropy, enhancing energy distribution.
- Irreversible Processes: Numerous natural processes are irreversible since they result in a net rise in entropy.
Misunderstandings to Address:
- Entropy may not always increase. In a closed system, it can remain stable.
- Entropy involves more than just disorder; it includes the energy distribution within the system.
Summary
- Grasping entropy is essential for understanding energy interactions in thermodynamics.
- Core Functionality: Entropy is key in anticipating phase changes and spontaneous reactions.
- Misconceptions and Realities: Dispel the notion that entropy simply denotes disorder, underscoring its broad role in energy distribution.
Enthalpy: Understanding the Heat Content
Key Concepts
- Enthalpy (H): Complete heat content within a system.
- Formula: , where is internal energy and is pressure-volume work.
- Units: Joules (J).
Key Definitions
- Enthalpy (H): Encompasses the total heat content in a system.
- Internal Energy (U): Sum of all energy within a system.
- Pressure-Volume Work (PV): Work executed on a system when volume alterations occur under pressure.
Understanding Enthalpy Changes
- Introduction to ΔH: ΔH quantifies heat uptake or release during reactions.
- Analogy: Similar to ice melting (heat uptake) or wood burning (heat release).
- Equation: , where indicates change in internal energy and represents work done.
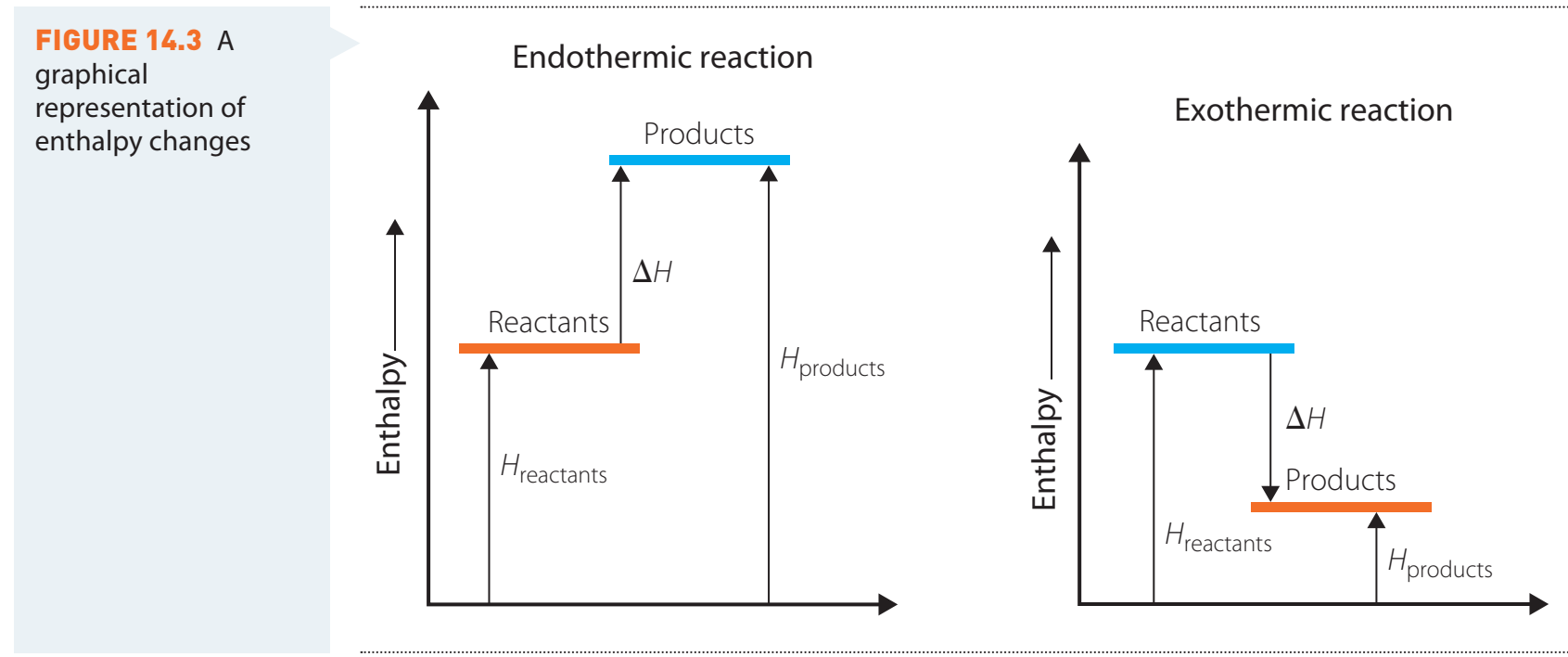
Exothermic vs. Endothermic Reactions
-
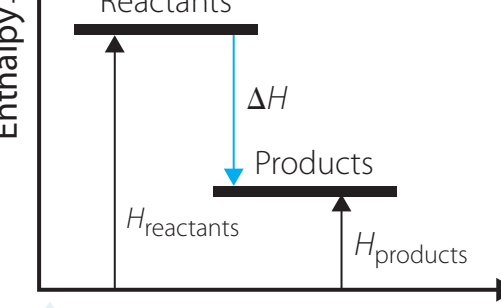
Exothermic Reactions:
- Definition: Process that releases heat, producing a negative ΔH.
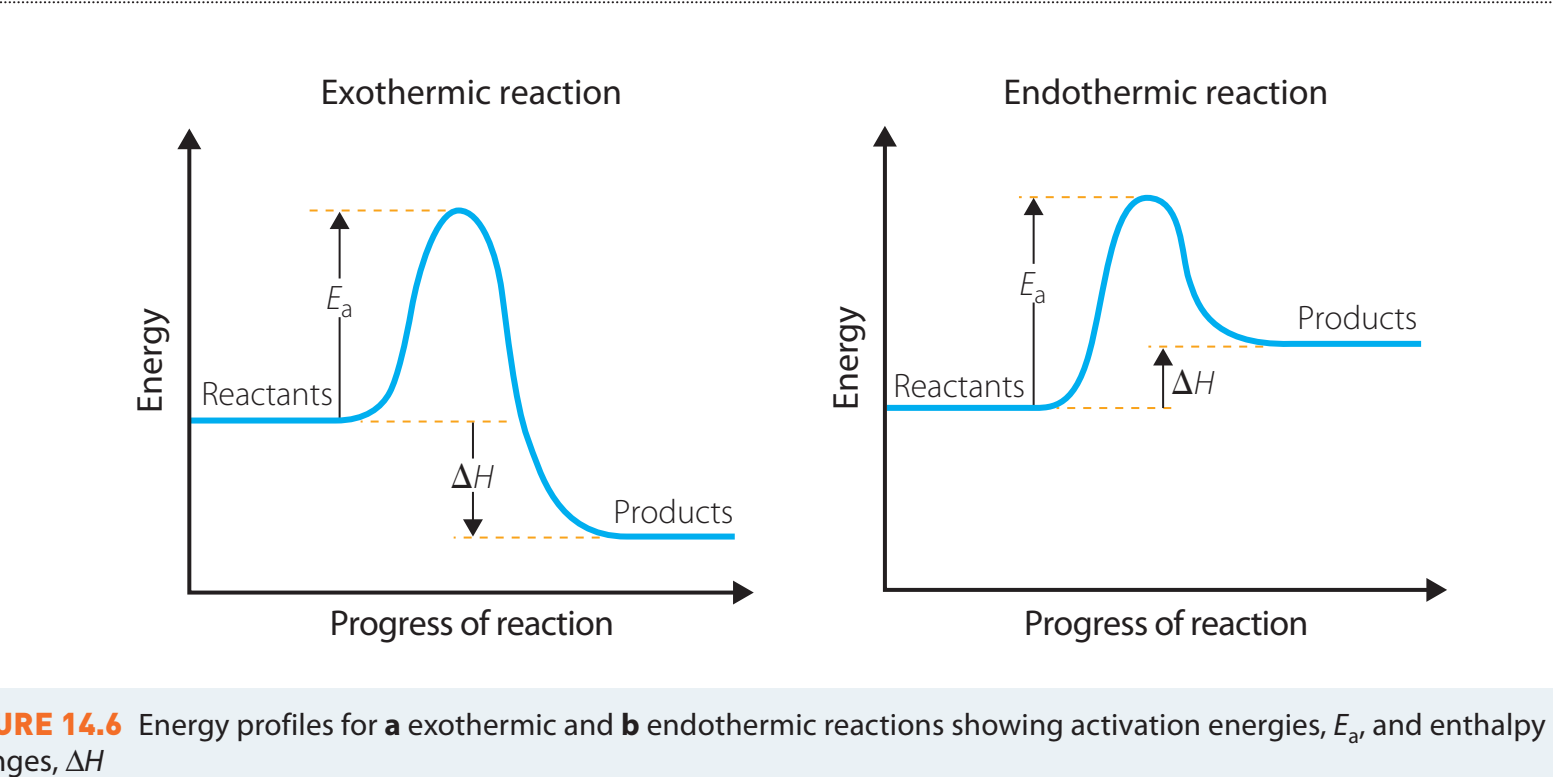
- Diagram Interpretation: Initial energy input represents activation energy, with a lower final state indicating heat release.
- Definition: Process that releases heat, producing a negative ΔH.
-
Endothermic Reactions:
- Definition: Processes that absorb heat, yielding a positive ΔH.
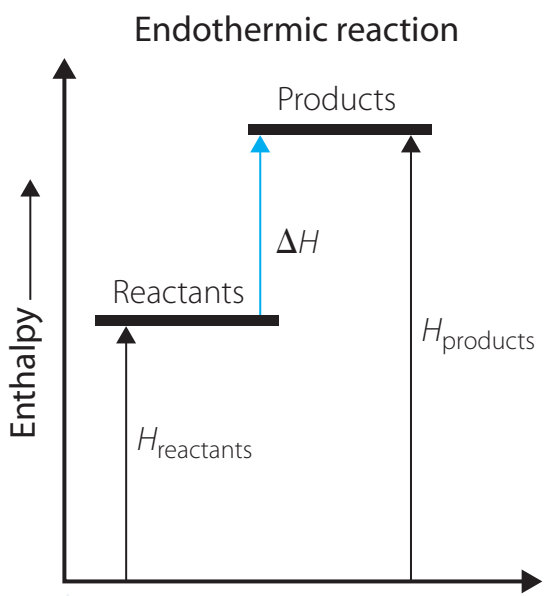
- Diagram Interpretation: A rising energy profile signifies an increase in heat.
- Definition: Processes that absorb heat, yielding a positive ΔH.
-
Standard State Conditions: Measurements taken at 298K, 1 atm.
Practical Examples and Calculations
- Example Walkthrough:
- Calculate ΔH using Hess's Law:
- Deconstruct the total reaction into stages.
- Utilise known ΔH values for each stage to ascertain total ΔH.
- Calculate ΔH using Hess's Law:
Combustion Example:
- Methane combustion:
- Given ΔH = -890.3 kJ/mol.
- Steps incorporate individual reactions of forming and .
Highlights and Key Points
- State Function Highlight: ΔH depends solely on initial and final states. Vital for studying thermodynamic properties.
- Exact Correspondence Callout: ΔH = q_p, indicating heat at constant pressure.
Misconceptions Clarified
- Common Errors:
- Confusing H with heat itself.
- Clarification: (heat under constant pressure).
- Assumptions:
- Erroneously linking higher enthalpy with stronger chemical bonds.
Tables/Charts
| Reaction | ΔH (kJ/mol) |
|---|---|
| Combustion of methane | -890.3 |
| Formation of water | -241.8 |
Table: Compare reactions under standard conditions showcasing ΔH values.
1. Introduction to Entropy vs. Enthalpy
- Entropy (S): Effect of energy dispersion within a system.
- Enthalpy (H): Comprehensive heat content at constant pressure.
- Core Question: How do these concepts differ and interact?
2. Entropy as Energy Dispersal
- Describes: The extent of energy dispersion within a system.
- Higher Entropy: Reflects extensive energy spread.
- Example: Gas expansion into a vacuum indicates rising entropy.
Entropy (S): Depicts energy spread within a system. More dispersed energy signifies increased entropy.
3. Enthalpy as Heat Content
- Measures: Total heat within a system under constant pressure.
- Includes: Internal energy and shifts in pressure-volume tasks.
- Examples: Cooling coffee and combustion illustrate enthalpy.
4. Comparing Energy Level Diagrams
- Diagrams Showcase: Exothermic vs. Endothermic processes.
- Energy Shifts: Visualised regarding both entropy and enthalpy.

Gibbs Free Energy: Combining Entropy and Enthalpy
Introduction
- Objective: Highlight how Gibbs Free Energy predicts reaction spontaneity by integrating entropy and enthalpy.
- Explanation: Gibbs Free Energy (G) calculates a balance between enthalpy (H) - total heat content, and entropy (S) - disorder within a system.
Fundamental Equation
- Formula Analysis:
- H: Enthalpy (Heat content)
- T: Temperature (Kelvin required)
- S: Entropy (Degree of disorder)
Temperature must be measured in Kelvin for accurate evaluations.
Derivation of
- Step-by-step Derivation:
- Begin with the equation .
- Connect , , and to energy and disorder changes.
- Recognise each component's effect on reaction viability.
Gibbs Free Energy and Spontaneity
- Reaction Predictions:
- Spontaneous:
- Equilibrium:
- Non-spontaneous:
- Temperature's Role: Temperature influences the TS term, affecting reaction spontaneity.
Illustrative Examples
- Example Calculations:
- Compute with given data: , , .
- Convert units from J to kJ: .
- Substitute into .
- Calculate: .
- Compute with given data: , , .
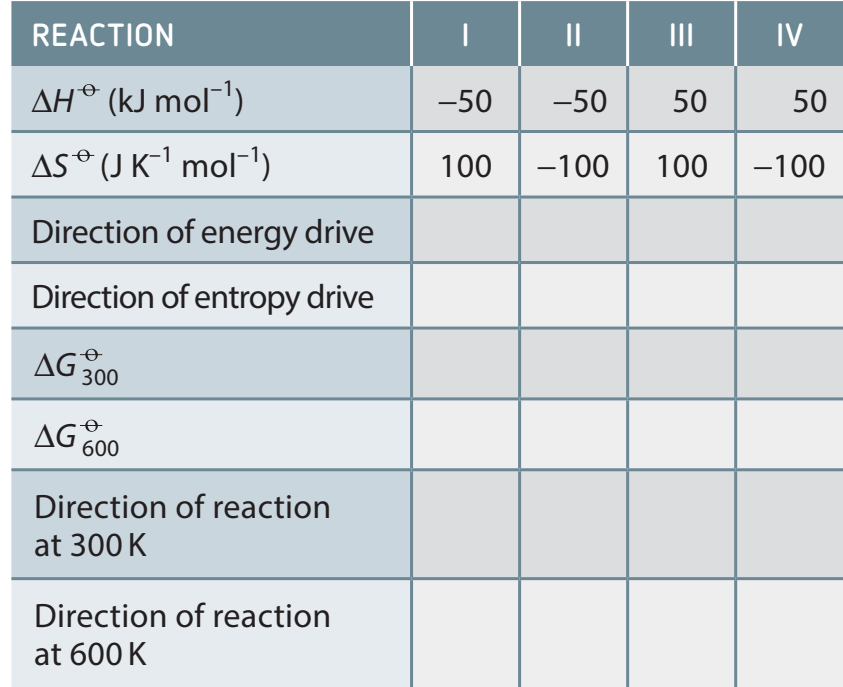
Importance of Temperature
- Scenario Analysis:
- Assess how temperature affects using graphical scenarios.
5. Interconnections
- Equation Used: Gibbs Free Energy, .
- Spontaneity Predictor: Assesses whether reactions occur spontaneously.
- Component Breakdown: assesses spontaneity, is heat-related, involves entropy.
- Worked Example: Evaluate to demonstrate reaction options and spontaneity.
Key Concepts and Highlights
- Entropy: Concerns the distribution of energy throughout systems.
- Enthalpy: Relates to a system's heat content under constant pressure.
- Potential Test Question: Given and values, compute and assess reaction spontaneity.
Clarifying Misconceptions
Introduction to Misconceptions
In thermodynamics, abstract concepts like entropy and enthalpy often lead to misunderstandings. Comprehending these terms is crucial for academic and practical applications.
Grasping thermodynamic complexities is crucial for correctly applying them in various scenarios.
Misconception about Entropy
- Misconception 1: Entropy as a measure of disorder.
- Clarification: Entropy measures energy dispersal rather than solely disorder.
- Example: Entropy increases when ideal gases mix, with no visible disorder change.
- Key Concept: Microstates and statistical mechanics explain entropy increases without evident disorder variations.
Microstates: Different possible configurations of a system. More microstates correspond to higher entropy.
- Misconception 2: ΔS is always positive.
- Clarification: ΔS can be negative, as in gas to liquid condensation where molecules arrange orderly.
Misconception about Enthalpy
-
Misconception 3: Enthalpy change (ΔH) concerns only heat.
- Clarification: ΔH includes internal energy changes and work, expressed as:
- Example: Pressure-volume work matters in gases; an expanding gas in a cylinder is a case.
-
Misconception 4: Standard enthalpy changes are consistent.
- Clarification: Temperature and pressure significantly impact standard enthalpy values.
Examples and Reinforcement
- Worked Example: Calculate ΔS for condensation. Given: Initial state = gas, Final state = liquid;
- Calculation: Use relevant formulas to detail changes.
- Explanation: A detailed guide presents practical application.
Worked Examples
Example 1: Entropy Calculation
Scenario: Ice melts into water at 0°C.
-
Steps:
- Calculate energy: J.
- Apply formula: .
- Interpretation: Importance of increased randomness as ice melts.
-
Result Statement: Entropy change is 22.05 J/K/mol.

Example 2: Enthalpy Change
Reaction: Combustion of methane.
- Steps:
- Compute energy for bond breakage/formation separately.
- Differentiate between energy usage and release.
- Analyse with Energy Profile Diagram.
Example 3: Gibbs Free Energy
Scenario: Water decomposition at 500K.
- Steps:
- Calculate using .
- Highlight impact on spontaneity with temperature variation.
- Real-life application in natural spontaneous reactions.

Practice Questions
Question 1: Entropy Change
Context: Gas expansion in a sealed container.
- Consider how volume change affects entropy: 10 L to 20 L at 298 K.
Solution:
- For an ideal gas expanding isothermally, entropy change can be calculated using:
- Given: L, L, K
- If we assume 1 mol of gas: J/K
Question 2: Enthalpy in Non-standard Conditions
Framework: Reaction at 2 atm pressure, 300 K.
- Calculate under these specific circumstances.
Solution:
- For a reaction at non-standard conditions, we must consider pressure effects.
- If the reaction involves gases, varies with pressure according to:
- If kJ/mol and mol: kJ/mol
Question 3: Gibbs Energy in Metabolic Process
Scenario: Feasibility in cells at 310K.
- Use Gibbs Free Energy equation to examine metabolic reaction conditions.
Solution:
- For a metabolic reaction with kJ/mol and J/K·mol:
- kJ/mol
- Since , the reaction is spontaneous at body temperature (310K).
- This demonstrates how some metabolic reactions become favourable at physiological temperatures despite being endothermic.
Tips and Tricks:
- Ensure unit consistency in computations.
- Interpret phase transitions accurately.
- Be attentive to sign errors in ΔH and ΔS.
- Incorporate tips into examples for thorough understanding.
500K+ Students Use These Powerful Tools to Master Entropy and Gibbs Energy For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
114 flashcards
Flashcards on Entropy and Gibbs Energy
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards12 quizzes
Quizzes on Entropy and Gibbs Energy
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes2 questions
Exam questions on Entropy and Gibbs Energy
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Entropy and Gibbs Energy
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Entropy and Gibbs Energy
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Entropy and Gibbs Energy you should explore
Discover More Revision Notes Related to Entropy and Gibbs Energy to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Entropy and Gibbs Free Energy
Entropy and Gibbs Free Energy
348+ studying
195KViews96%
114 rated
Entropy and Gibbs Free Energy
Entropy and Gibbs Free Energy
239+ studying
197KViews