Photo AI
Last Updated Sep 24, 2025
Gibbs Free Energy Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Gibbs Free Energy quickly and effectively.
400+ students studying
Gibbs Free Energy
Introduction to Gibbs Free Energy
- Definition of Gibbs Free Energy (G):
- Denotes the maximum reversible work a system can perform at constant temperature and pressure.
- Energy available for chemical transformations excluding expansion work.
Gibbs Free Energy: Essential in assessing available energy under constant conditions.
Predicting Reaction Outcomes
- Conditions Indicated by ΔG:
- Negative ΔG: Reaction is spontaneous.
- Zero ΔG: Reaction is at equilibrium.
- Positive ΔG: Reaction is non-spontaneous.
Spontaneity Conditions:
- ΔG < 0: Spontaneous by nature.
- ΔG = 0: System reached equilibrium.
- ΔG > 0: Requires an external energy source.
Formula Overview
- Formula:
- ΔG: Change in Gibbs Free Energy
- ΔH: Change in enthalpy (heat content)
- T: Absolute temperature (Kelvin)
- ΔS: Change in entropy (measure of disorder)
Worked Examples
Example Calculation
Given:
- Reaction at 298 K
- ΔH = -10 kJ/mol
- ΔS = -50 J/mol·K
Let's calculate the change in Gibbs Free Energy:
- First, convert ΔH to joules: ΔH = -10,000 J/mol
- Apply the formula :
Interpretation: Since ΔG > 0, the reaction is non-spontaneous.
Understanding Reaction Spontaneity
Criteria for Spontaneity
Spontaneous Process: A process is spontaneous if , indicating thermodynamic favourability. This is unrelated to the reaction rate.
Temperature's Role (T)
- Influence on : Temperature can impact the spontaneity of a reaction using the equation .
Misconceptions
Speed vs. Spontaneity
- Gibbs Free Energy () signifies if a reaction is possible, but not its speed.
Spontaneity does not equal speed. It denotes thermodynamic feasibility, distinct from kinetic factors.
Enthalpy ()
Enthalpy: Total heat content of a system at constant pressure.
Processes:
- Endothermic:
- Absorbs heat ().
- Example: Melting of ice, requiring heat absorption for the solid-to-liquid transition.
- Exothermic:
- Releases heat ().
- Example: Combustion of a fuel, releasing energy in the form of heat.
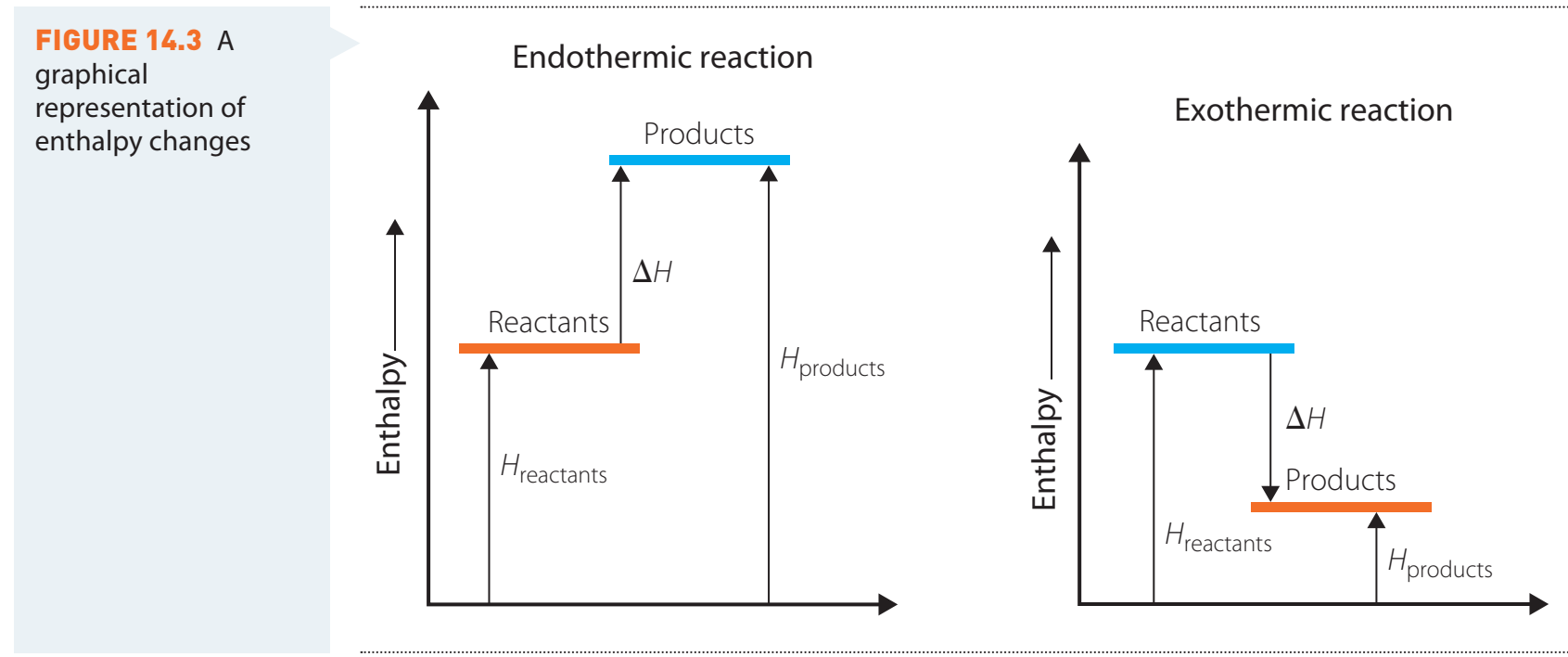
Entropy ()
Entropy: A measure of disorder or randomness within a system.
Examples:
- Phase Changes:
- Moving from solid liquid gas increases .
- Example: Water transitioning from ice to steam, reflecting increasing disorder.
- Dissolution:
- Salt dissolving in water causes an increase in , indicating increased randomness as ions separate.
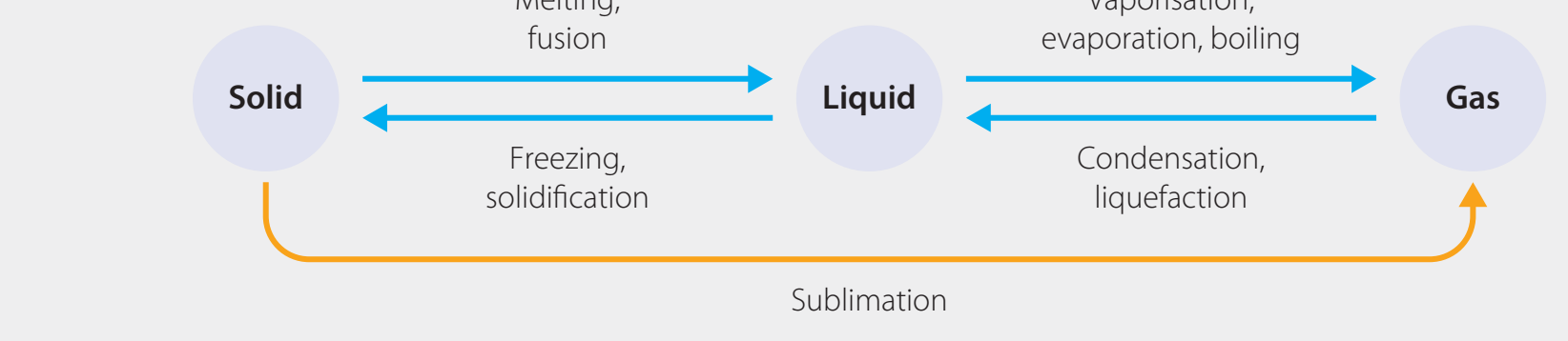
Clarification Note: Entropy relates to energy arrangement and distribution, not simply chaos.
Practice Problems
-
Example 1:
- Assess conditions for reactions spontaneous at low temperatures.
- Given:
- Calculate:
- (Spontaneous)
- (Non-spontaneous)
- Given:
- Assess conditions for reactions spontaneous at low temperatures.
-
Example 2:
- Demonstrate non-spontaneous reactions at varying temperatures.
- Given:
- Analysis:
- At any temperature:
- Since both terms are positive, will always be positive
- Therefore, the reaction is non-spontaneous at all temperatures
- Given:
- Demonstrate non-spontaneous reactions at varying temperatures.
Visual Aids
Annotated Diagrams
- Phase Change Diagram:
- Purpose: Showcases how entropy and enthalpy vary during transitions like melting and boiling.
- Annotations:
- X-axis: Reflects temperature variations.
- Y-axis: Indicates entropy and enthalpy changes.
- Real-World Examples:
- Boiling Water: Demonstrates increased entropy as water converts to steam.
- Melting Ice: Exhibits entropy change as solid transitions to liquid.

- Graphs for Entropy, Enthalpy, and Gibbs Free Energy:
- Description: Outlines the interaction between , , and .

Addressing Misconceptions
Misconceptions
- "Entropy equals chaos":
-
infoNote
Correction: Entropy is a metric, not chaos; it measures disorder.
-
- "Spontaneity means fast":
-
infoNote
Correction: Spontaneity signifies thermodynamic favourability, not speed.
-
- "Negative equals rapid reaction":
-
infoNote
Correction: A negative indicates potential onset, not reaction speed.
-
Importance of Catalyst
- Catalyst Significance:
- Vital in both academic study and industrial applications.
- Example: Enzymes act as biological catalysts, critical for accelerating metabolic reactions.
Exam Tips
- Spontaneity vs. Speed: Avoid confusing speed with spontaneity. Catalysts expedite reactions but do not alter Gibbs Free Energy.
Gibbs Free Energy (∆G): Predicts reaction spontaneity by calculating energy changes due to chemical processes.
500K+ Students Use These Powerful Tools to Master Gibbs Free Energy For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
114 flashcards
Flashcards on Gibbs Free Energy
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards12 quizzes
Quizzes on Gibbs Free Energy
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes2 questions
Exam questions on Gibbs Free Energy
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Gibbs Free Energy
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Gibbs Free Energy
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Gibbs Free Energy you should explore
Discover More Revision Notes Related to Gibbs Free Energy to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Entropy and Gibbs Free Energy
Entropy and Gibbs Free Energy
399+ studying
195KViews96%
114 rated
Entropy and Gibbs Free Energy
Entropy and Gibbs Free Energy
239+ studying
194KViews