Photo AI
Last Updated Sep 24, 2025
Entropy and Gibbs Free Energy Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Entropy and Gibbs Free Energy quickly and effectively.
429+ students studying
Entropy and Gibbs Free Energy
Introduction
- Entropy: A quantitative measure of disorder or randomness within chemical systems.
- Essential for comprehending natural processes and assessing reaction feasibility and spontaneity.
- Relevant to pre-college chemistry curricula.
Historical Context
- Ludwig Boltzmann:
- Established the statistical mechanics perspective on entropy.
- Developed the Boltzmann entropy equation: , where represents entropy, is the Boltzmann constant, and signifies the number of microstates.
- Rudolf Clausius:
- Initially introduced the concept of entropy.
- Simplified Timeline:
- 1850s: Introduction of entropy by Clausius.
- Late 1800s: Contributions by Boltzmann in statistical fields.
- 20th Century: Expanded applications of entropy.

Did You Know?
Boltzmann's groundbreaking work significantly impacted fields outside physics, including information theory.
Fundamental Principles
- Second Law of Thermodynamics:
- Systems in nature spontaneously tend toward higher entropy states.
- Statistical Mechanics:
- Microstates: Specific states of a system.
- Macrostate: Cumulative observable state.
- Relevant Formula:
Example Problem
- Determine entropy change () for a system transitioning from 1 to 100 microstates.
- Solution:
A thorough grasp of Boltzmann's Equation is essential for comprehending entropy concepts.
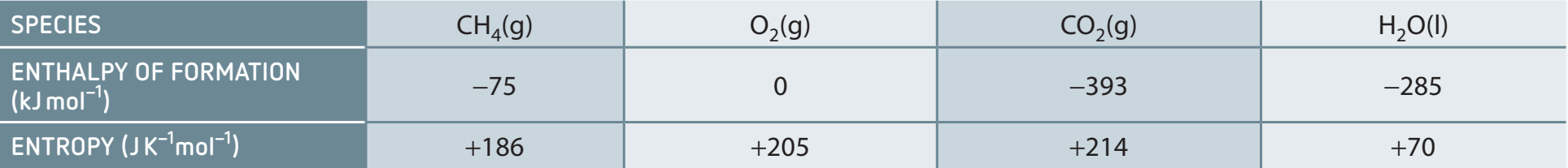
Entropy in Thermodynamics
- Role and Significance:
- Fundamental to understanding energy distribution.
- Crucial for predicting chemical equilibrium.
- Enthalpy vs. Entropy:
- Enthalpy: Heat energy exchange at constant pressure.
- Entropy: Distribution of energy and disorder.
Guidelines for Predicting Entropy Changes
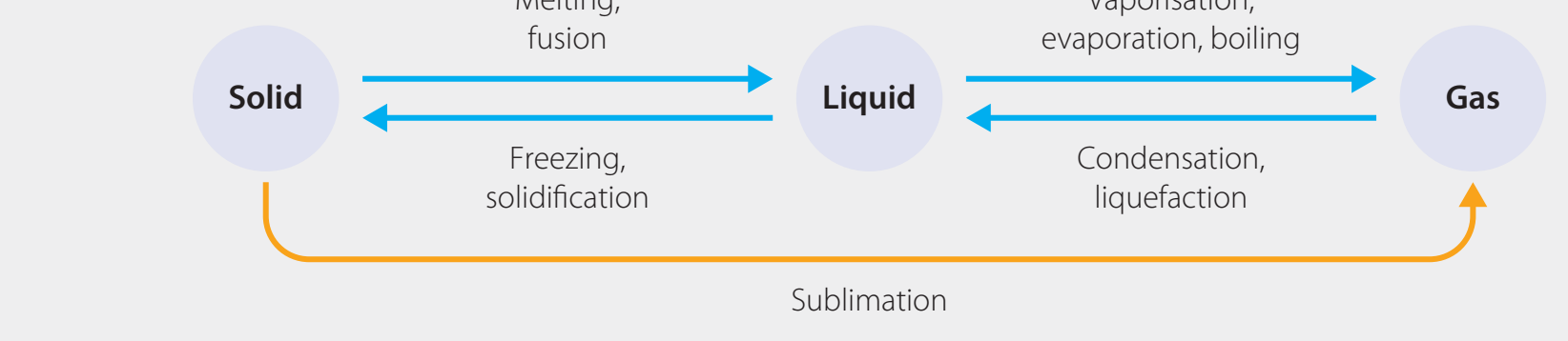
States of Matter
- Entropy Hierarchy:
- Gases have higher entropy than liquids, which have higher entropy than solids.
Understanding the Entropy Hierarchy:
- Gas > Liquid > Solid
- Transitions causing increased entropy:
- Solid ➔ Liquid: Example, ice melting.
- Liquid ➔ Gas: Example, water boiling.
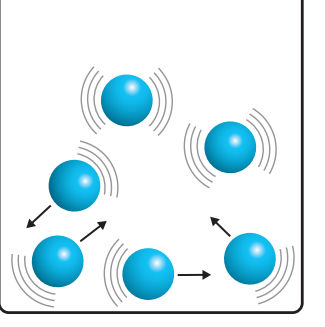
Moles of Gas
- Increasing the number of gas moles correlates with increased entropy.
Crucial Insight:
- More gas = Increased entropy
Practical Examples
Perfumed Diffusion in Water
- Step-by-step Process:
- Add perfume to water.
- Observe molecules disperse from the concentrated point.
- Notice the increase in disorder.

State Changes (Solid, Liquid, Gas)
- Ice Melting: Molecules gain increased freedom, leading to greater disorder.
- Water Boiling: Molecules disperse widely, indicating an increase in entropy.
Misconceptions
-
Entropy Not Merely Chaos:
- The concept is broader, involving probabilities of microstates.
-
Entropy and Spontaneity:
- Positive entropy alone does not ensure spontaneity.
- Gibbs Free Energy, , is essential for predicting reaction spontaneity.
-
Exam Tips:
- Grasp entropy through practical examples like gas expansion.
Mastering Gibbs Free Energy is crucial for resolving misunderstandings about entropy and spontaneity.
Conclusion
Addressing misconceptions and understanding entropy fundamentals are vital for excelling in thermodynamics and preparing students for future scientific endeavours. This comprehensive yet approachable note supports pre-college students in gaining a solid understanding of entropy.
500K+ Students Use These Powerful Tools to Master Entropy and Gibbs Free Energy For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
114 flashcards
Flashcards on Entropy and Gibbs Free Energy
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards12 quizzes
Quizzes on Entropy and Gibbs Free Energy
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes2 questions
Exam questions on Entropy and Gibbs Free Energy
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Entropy and Gibbs Free Energy
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Entropy and Gibbs Free Energy
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Entropy and Gibbs Free Energy you should explore
Discover More Revision Notes Related to Entropy and Gibbs Free Energy to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Entropy and Gibbs Free Energy
Entropy and Gibbs Free Energy
378+ studying
187KViews