Photo AI
Last Updated Sep 24, 2025
Alcohols Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Alcohols quickly and effectively.
326+ students studying
Alcohols
Introduction
Understanding the chemistry of alcohols is essential in various scientific disciplines due to their numerous applications and the unique characteristics imparted by their hydroxyl (-OH) functional group.
Key Term: Alcohols: Organic compounds characterised by the presence of a hydroxyl group (-OH).
- Significance: Alcohols are indispensable in industries such as cosmetics, medicine, and fuel due to their solvent abilities and antiseptic qualities.
- General Formula: R-OH, where R denotes an alkyl group (e.g., methyl (CH₃) or ethyl (C₂H₅)).
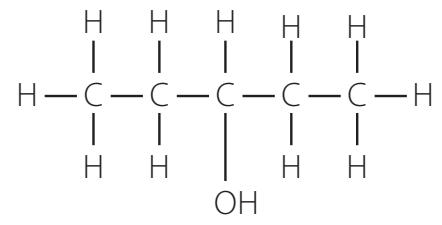
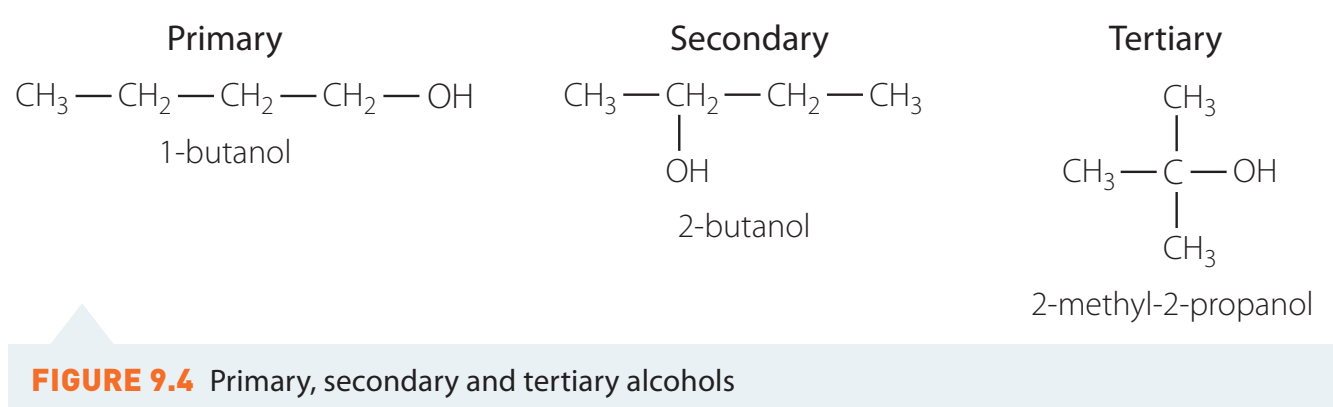
Structural Formulae of Alcohols
Alcohols are classified based on the carbon atom associated with the -OH group:
- Primary: Bound to one other carbon atom.
- Secondary: Bound to two other carbon atoms.
- Tertiary: Bound to three other carbon atoms.
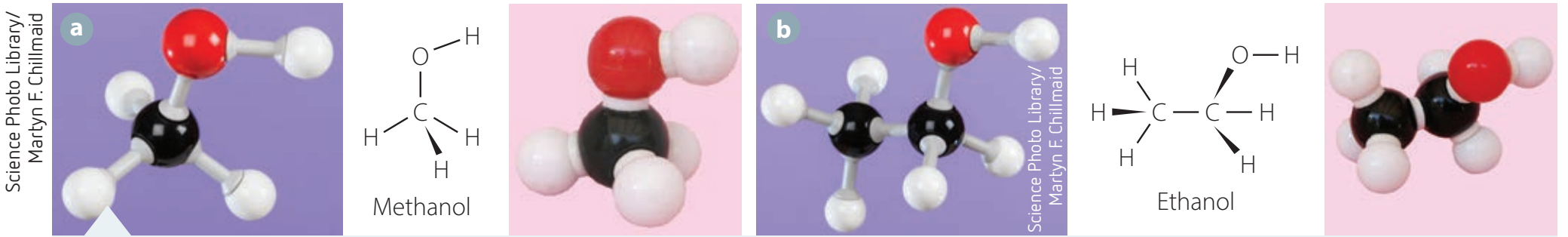
Primary Alcohols
Examples: Methanol (CH₃OH), Ethanol (C₂H₅OH)
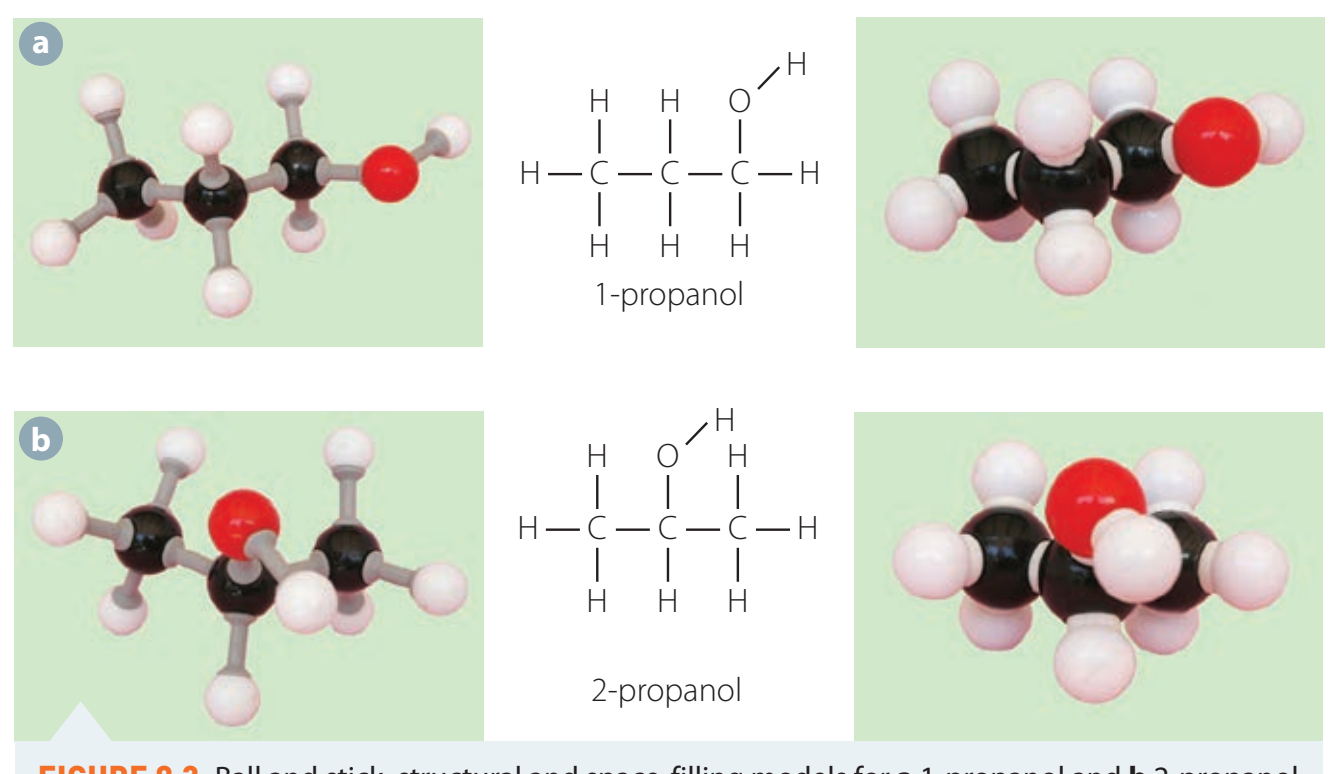
Secondary Alcohols
Example: Isopropanol [(CH₃)₂CHOH]
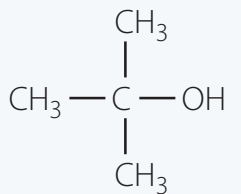
Tertiary Alcohols
Example: Tert-Butanol [(CH₃)₃COH]
Key Term: Alkyl group: A hydrocarbon group represented by R in formulas.
Properties of Alcohols
Boiling Points and Solubility
-
Hydrogen Bonding: Alcohols exhibit higher boiling points than hydrocarbons, as shown in the table below:
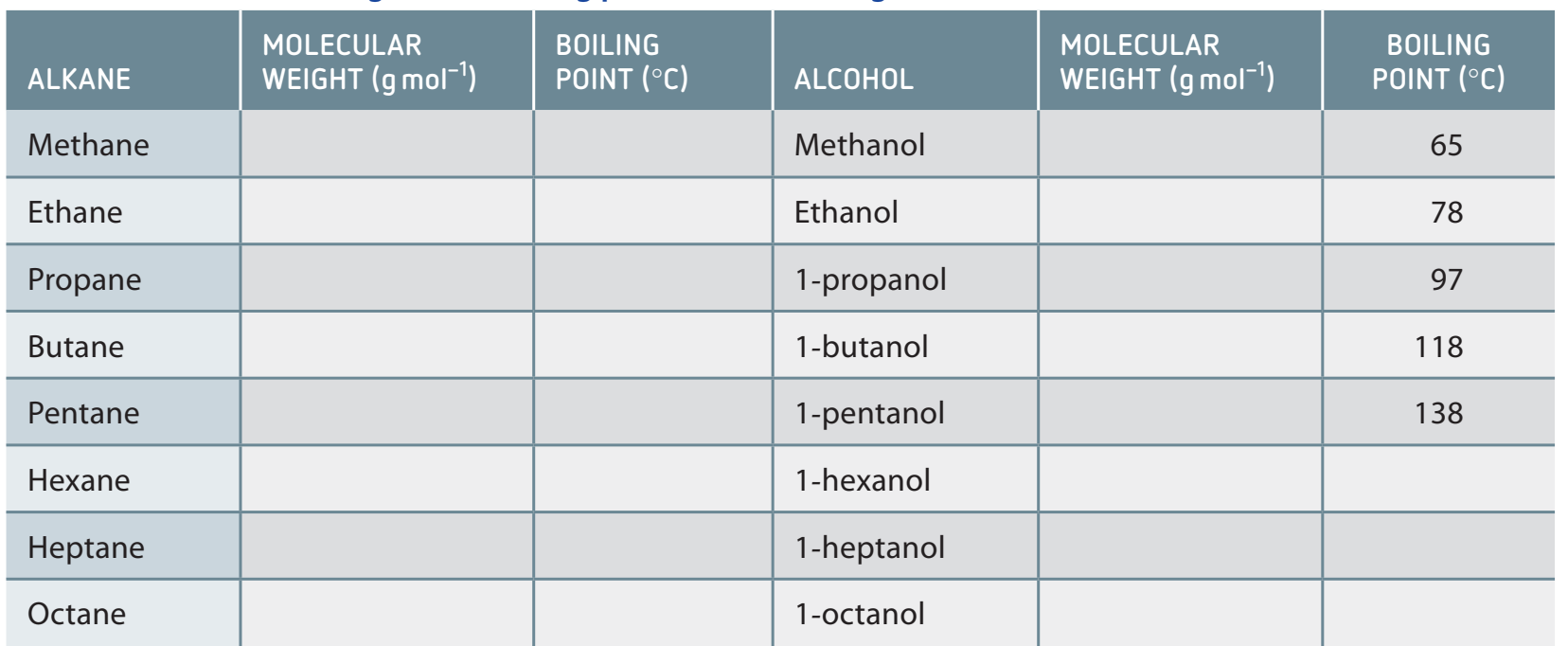
-
Solubility: Decreases as the chain length increases, due to a reduction in polar interactions with water.
Impact of Chain Length
-
Boiling Points: Increase with chain length due to stronger van der Waals forces.

Comparison of Alcohol Types
- Primary Alcohols: Exhibit higher boiling points due to less branching.
- Tertiary Alcohols: Display the lowest boiling point due to increased branching.
- Alcohols: Present distinct intermolecular forces such as van der Waals, dipole-dipole, and hydrogen bonding, influencing their physical states and reactivity.
Reactions of Alcohols
Alcohols are defined by their reactive hydroxyl (OH) group.
Combustion Reactions
- Complete Combustion: Results in carbon dioxide and water, releasing energy.
- Methanol example:
- Incomplete Combustion: Produces carbon monoxide (CO) and soot, posing risks such as pollution.
Dehydration Reactions
- Convert alcohols to alkenes by removing water, using acid catalysts.
Study reaction mechanisms to comprehend conditions like acid strength and reaction pathways.
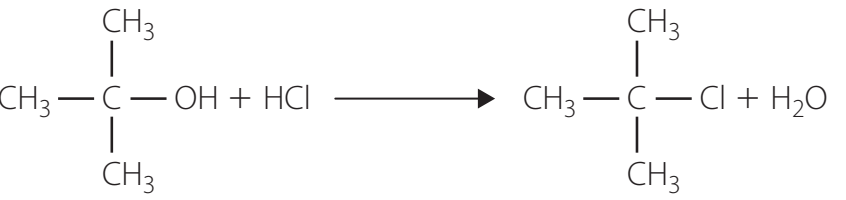
Substitution Reactions
- React with Hydrohalic Acids (HX): Forming alkyl halides, the reactivity order is tertiary > secondary > primary.
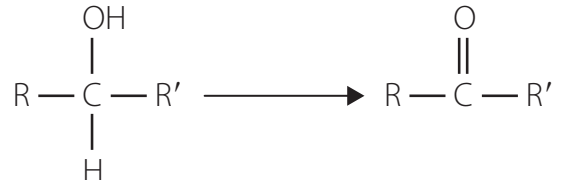
Oxidation Reactions
- Primary Alcohols: Convert to aldehydes or carboxylic acids with oxidising agents.
- Secondary Alcohols: Convert to ketones, not further oxidisable.
- Tertiary Alcohols: Resistant to oxidation.

Alcohol Production Processes
Substitution Reactions
- Halogens are replaced by hydroxyl groups with the use of catalysts.
Fermentation
- Biological conversion of sugars into ethanol.
- Example: Glucose () to ethanol ()
- Typical conditions: Temperature 30-35°C, pH 4-5
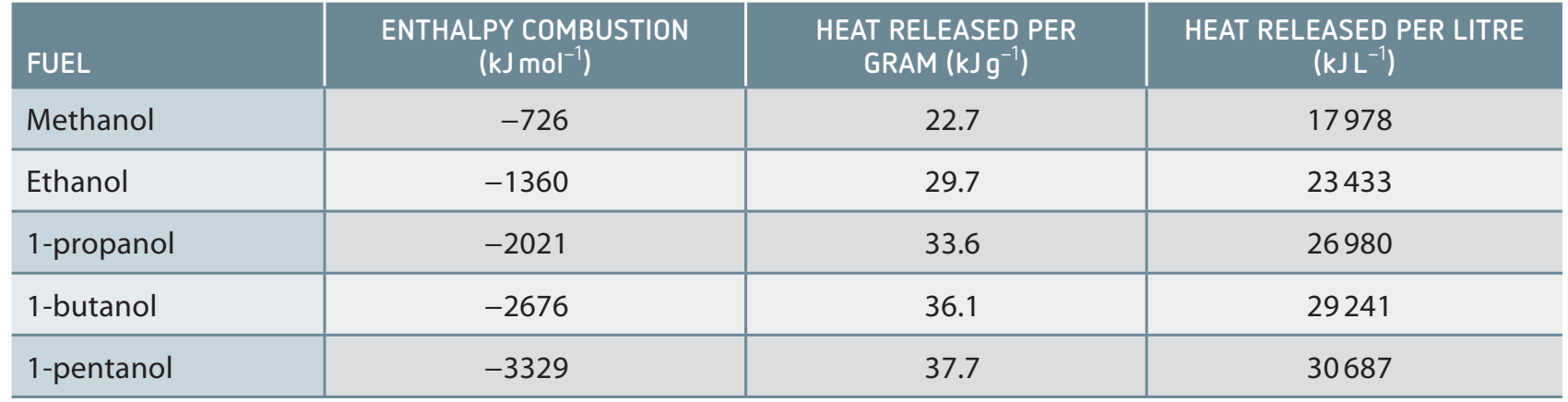
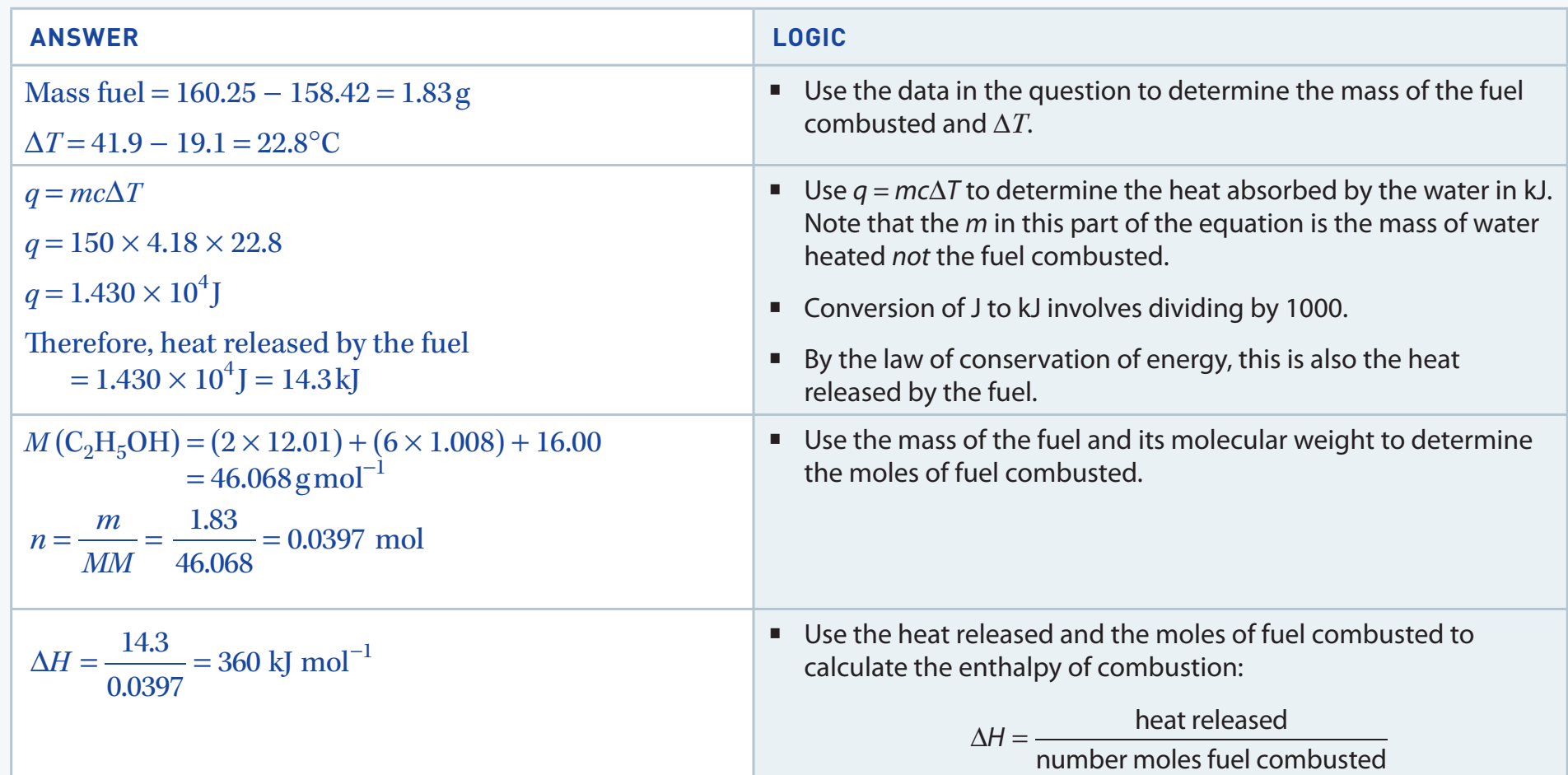
Enthalpy of Combustion
Definition: The energy change when one mole of alcohol fully combusts.
- Calorimetry: This technique measures energy changes, crucial for assessing fuel efficiency.
Sample Calculations
- Formula: (where q is the heat absorbed, n is moles burnt)
Worked Example:
If burning 0.05 moles of ethanol releases 150 kJ of heat, what is the enthalpy of combustion?
Solution:
The enthalpy of combustion for ethanol is -3000 kJ/mol.
Safety, Environmental Impact, and Industrial Significance
- Safety: Handle alcohols, such as ethanol, with care due to their flammable nature.
- Environmental Impact: Biofuels like bioethanol offer a renewable alternative to fossil fuels, promoting sustainability.
- Role in Modern Industry: Used extensively as solvents, fuels, and in sanitation.
Overview of Oxidation in Organic Chemistry
Oxidation: Plays a central role in transforming alcohols into more complex compounds, which are crucial for the chemical industry.
- Alcohol Conversion: Primary alcohols convert into aldehydes/carboxylic acids, secondary into ketones, while tertiary show resistance to oxidation.
By understanding alcohols and their diverse reactions, students gain valuable insights into an essential class of compounds that significantly impact both scientific fields and industrial processes.
500K+ Students Use These Powerful Tools to Master Alcohols For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
299 flashcards
Flashcards on Alcohols
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Alcohols
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Alcohols
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Alcohols
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Alcohols
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alcohols you should explore
Discover More Revision Notes Related to Alcohols to Deepen Your Understanding and Improve Your Mastery
