Photo AI
Last Updated Sep 24, 2025
Aldehydes Chemistry Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Aldehydes Chemistry quickly and effectively.
362+ students studying
Aldehydes Chemistry
Introduction to Aldehydes
Aldehydes are a fundamental class of compounds in organic chemistry. Their intrinsic structure and reactivity are essential for comprehending chemical reactions in Year 12 studies. Aldehydes are prevalent in both natural processes and synthetic chemistry.
Definition and Characteristics
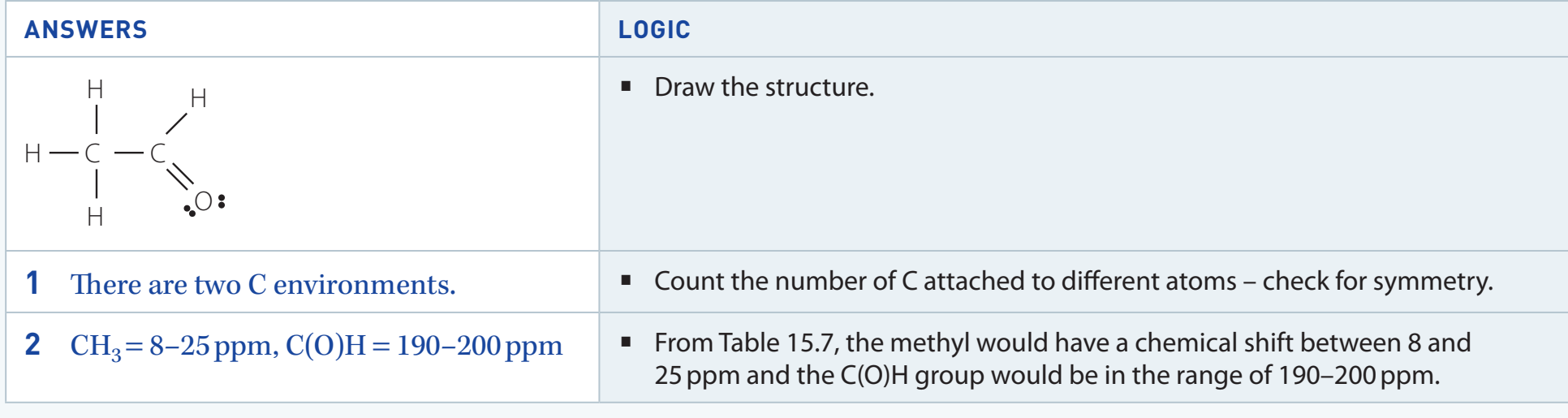
- Aldehydes: Organic compounds characterised by a terminal group. This specific structure significantly influences their chemical behaviour.
- Significance: The presence of a terminal carbonyl group imparts unique properties to aldehydes, facilitating a variety of chemical reactions.
- Functional Group Characteristics:
- The carbonyl group in aldehydes is planar, which determines their interactions with other molecules.
- The electron-deficient carbon atom readily undergoes nucleophilic addition reactions.
Structural Differences Between Aldehydes and Ketones
- Aldehydes:
- Feature a terminal carbonyl group .
- Ketones:
- Possess a carbonyl group embedded within the carbon chain.
- Impact:
- These structural distinctions markedly affect their chemical properties and reactivity.
Historical Context
- Evolution Over Time: Early research by chemists such as Friedrich Wöhler has informed modern methodologies.
- Current Significance: Present applications in organic synthesis owe much to the foundational discoveries regarding aldehydes.
Stay tuned for upcoming discussions on pivotal aldehyde reactions including Tollens' and Fehling's tests.
Introduction to Aldehyde Nomenclature
Aldehydes are distinguishable by their characteristic '-al' suffix, indicating the existence of a terminal carbonyl group. IUPAC nomenclature is crucial as it offers a reliable framework for uniform and precise chemical naming.
Systematic IUPAC Rules
To systematically name aldehydes, follow these guidelines:
- Identify the longest carbon chain that incorporates the aldehyde carbon as the first carbon.
- Locate and denote substituents on this chain, ensuring correct positioning and numbering.
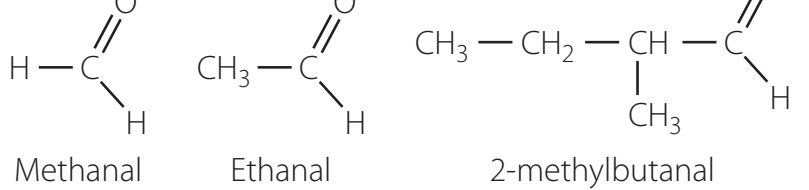
Worked Example:
- is named ethanal: The longest chain comprises two carbons, commencing from the aldehyde group.
Common and Systematic Names
Understanding both common and systematic names enhances chemical literacy:
- Formaldehyde/Methanal
- Acetaldehyde/Ethanal
Each naming convention serves distinct purposes: IUPAC names for formal use, common names in laboratory settings and historical contexts.
Mastering these strategies enhances chemical nomenclature accuracy and minimises errors.
Introduction to Physical Properties
Aldehydes possess distinct physical properties due to the presence of the carbonyl group, influencing their boiling points, solubility, and polarity.
Carbonyl Group: A functional group characterised by a carbon atom double-bonded to an oxygen atom .
Boiling Points of Aldehydes
- Polar Carbonyl Group: Confers higher boiling points compared to hydrocarbons with similar molar mass.
- Example Molecules: Formaldehyde and acetaldehyde exhibit higher boiling points relative to hydrocarbons such as ethane.
- Lack of Hydrogen Bonding: Results in aldehydes having lower boiling points than alcohols.
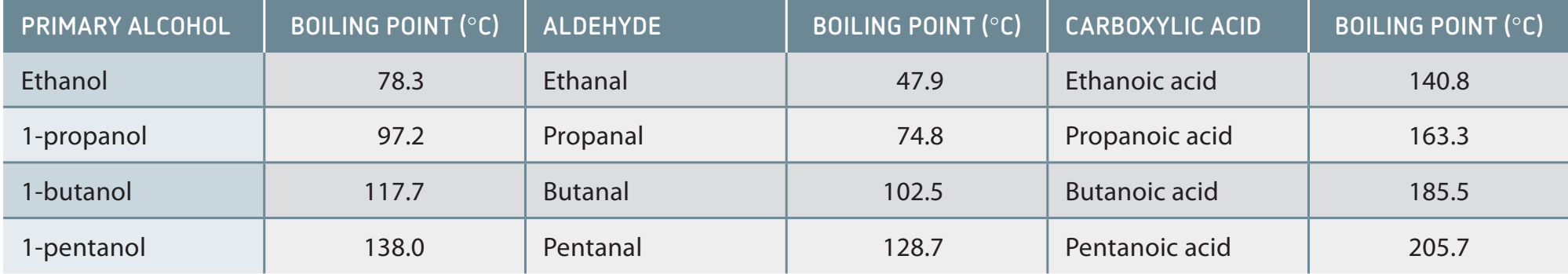
Solubility Patterns
- Water Solubility: Smaller aldehydes like formaldehyde are water-soluble due to hydrogen bonding.
- Effect of Molecular Size: Larger aldehydes exhibit decreased water solubility but dissolve well in non-polar solvents.
Role of Polarity
- Dipole-Dipole Interactions: Aldehydes experience these interactions, setting them apart from non-polar substances.
- Dipole interactions influence the behaviour of aldehydes in mixed solutions.
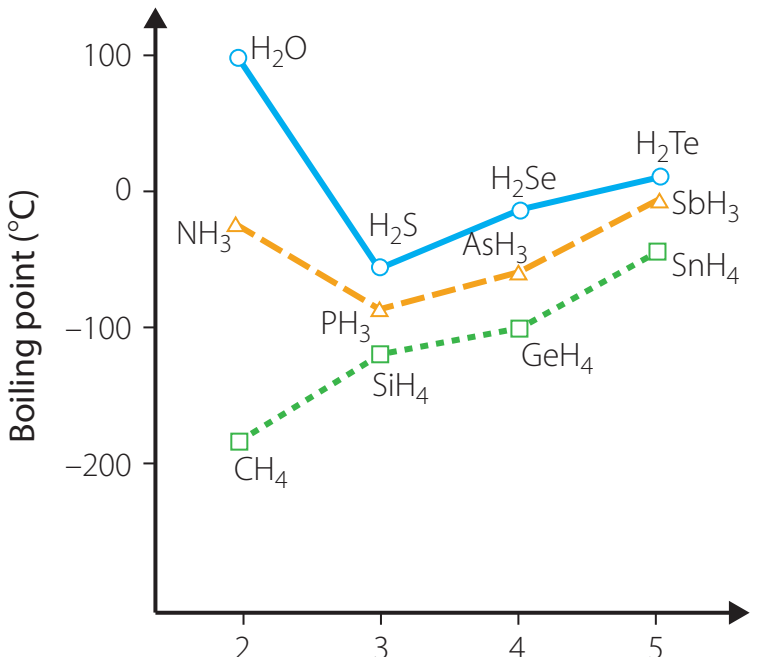
Visualisation and Trends
- Boiling Point vs. Molecular Weight: Graph depicts the correlation between molecular weight and boiling point.
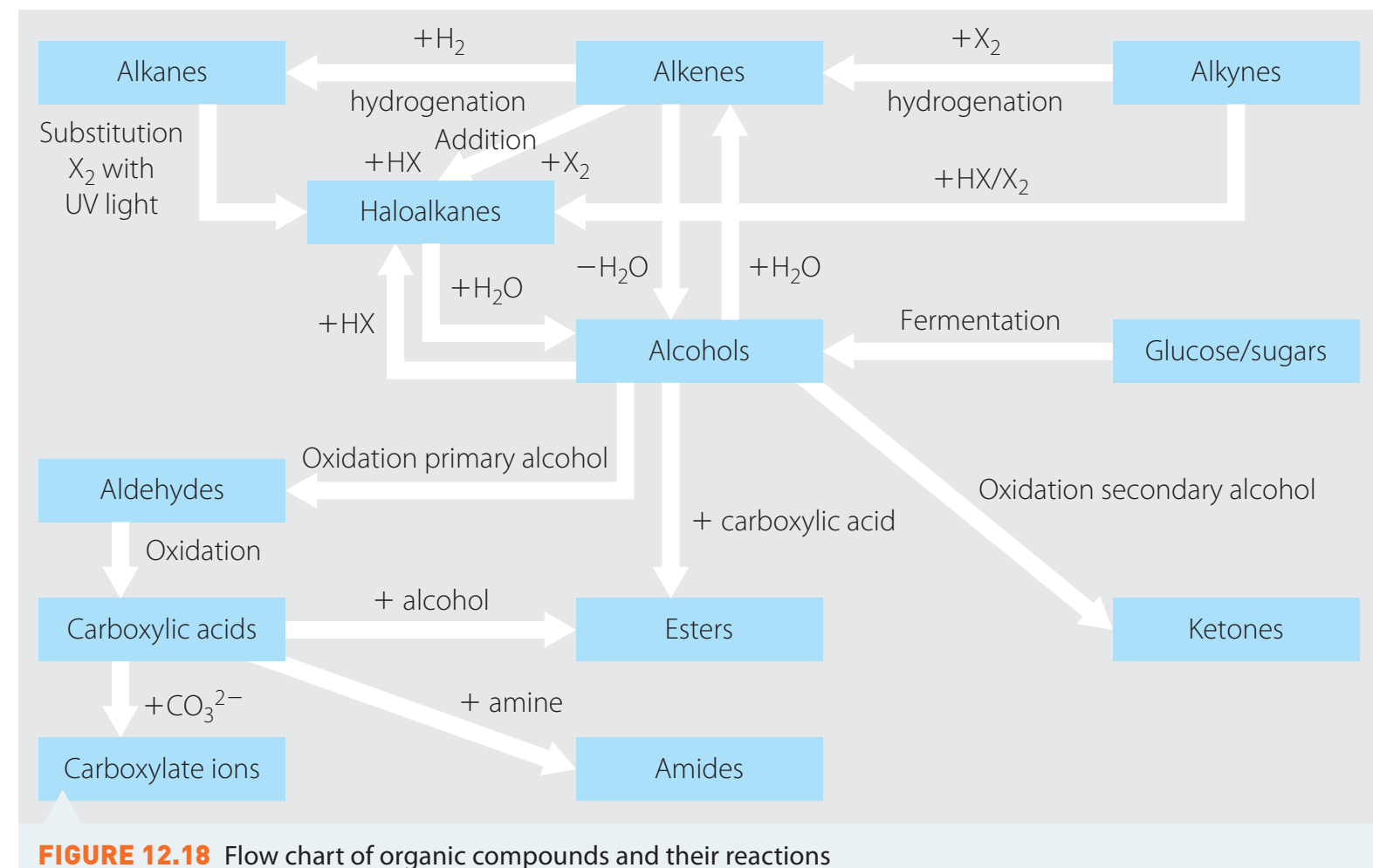
Overview of Aldehyde Reactions
Aldehydes are highly reactive due to the carbonyl group, which serves as a unique reactive site.
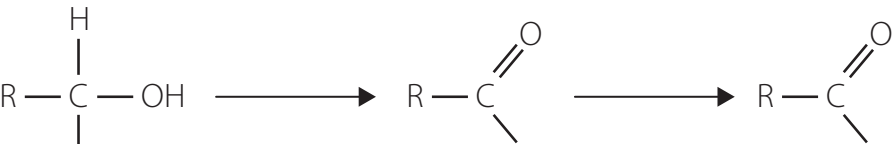
Oxidation Reactions
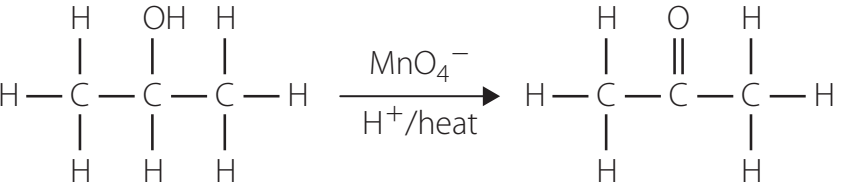
Tollens' Test
- Objective: Identify aldehydes through the formation of a silver mirror.
- Reagents: Ammoniacal silver nitrate solution.
- Procedure: Requires clean test tubes, addition of reagent, and gentle heating.
- Chemical Equation:
Fehling's Test
- Objective: Differentiate aldehydes from ketones by a brick-red precipitate.
- Reagents: Fehling's solution containing copper ions.
- Chemical Equation:
Introduction to Aldehyde Synthesis
Aldehydes play a pivotal role in industrial and laboratory synthesis due to their versatile chemical behaviour.
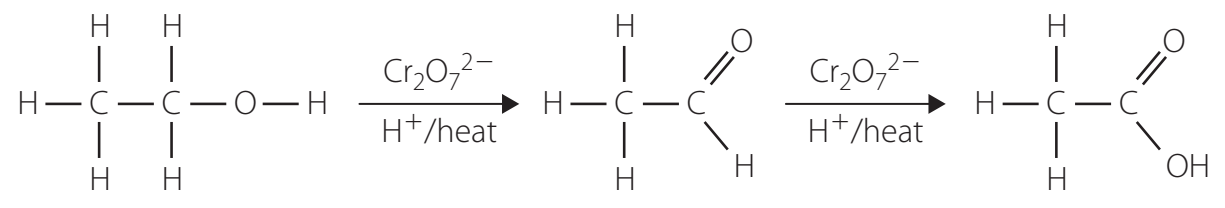
Oxidation of Primary Alcohols
- Importance: Key process for converting alcohols into aldehydes.
- Reagents: Pyridinium Chlorochromate (PCC).
- Chemical Equation: compounds
Glossary
Nucleophilic Addition
- Definition: A reaction in which a nucleophile forms a bond with a positive or partially positive atom.

Oxidation
- Definition: This process involves electron loss, typically with oxygen addition or hydrogen removal.
Consistently revise these concepts to build a comprehensive understanding of aldehydes in preparation for your exams.
500K+ Students Use These Powerful Tools to Master Aldehydes Chemistry For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
299 flashcards
Flashcards on Aldehydes Chemistry
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Aldehydes Chemistry
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Aldehydes Chemistry
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Aldehydes Chemistry
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Aldehydes Chemistry
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Aldehydes Chemistry you should explore
Discover More Revision Notes Related to Aldehydes Chemistry to Deepen Your Understanding and Improve Your Mastery
