Photo AI
Last Updated Sep 24, 2025
Carboxylic Acids Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Carboxylic Acids quickly and effectively.
371+ students studying
Carboxylic Acids
Introduction to Carboxylic Acids
Definition
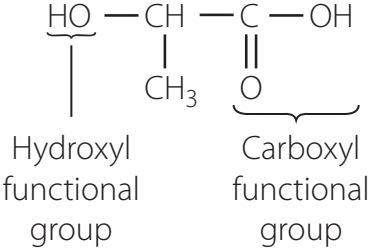
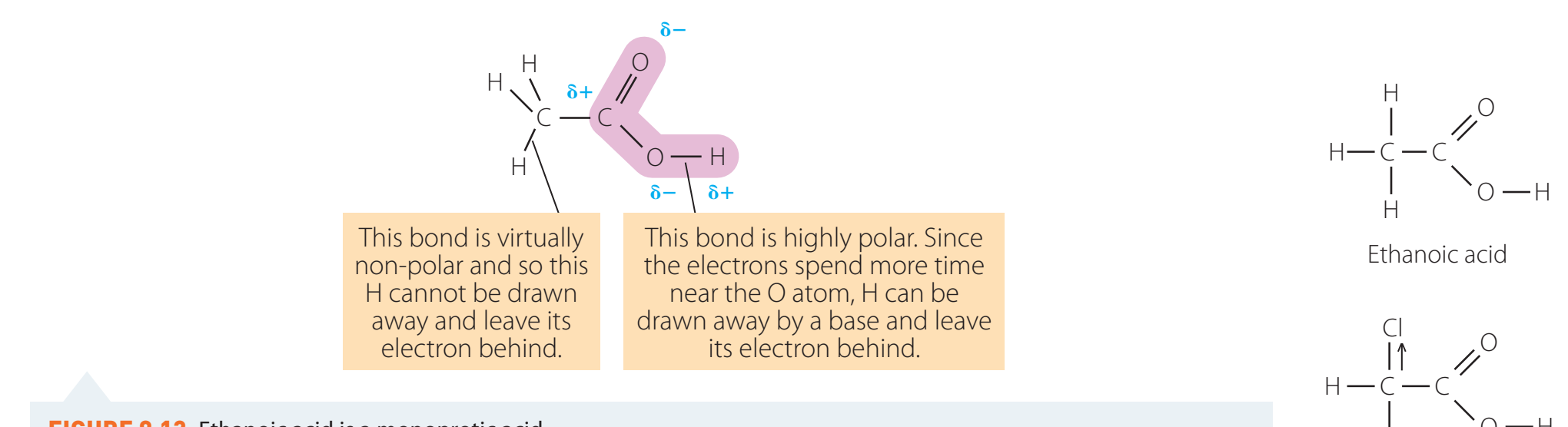
Carboxylic acids are organic compounds identified by the presence of a carboxyl group (—COOH).
- General formula: R-COOH
- R symbolises a hydrocarbon chain or group.
- Example: If R is a methyl group (CH₃), the compound is acetic acid.
Carboxyl Functional Group Structure
- Carbonyl group (C=O): Polar due to oxygen's higher electronegativity.
- Hydroxyl group (—OH): Enhances acidity due to bond polarity.
- Together, these form the polar carboxyl group (—COOH).
Polarity Effects:
- Improves solubility in water, attributable to the group's polar nature.
- Encourages acidic behaviour, allowing hydrogen dissociation.
- Bond angles and sp² hybridisation in the carbonyl group increase reactivity.
Enhance Student Understanding
-
Common Misconception: Hydroxyl groups in carboxylic acids are distinct from those in alcohols due to the presence of the carbonyl group.
-
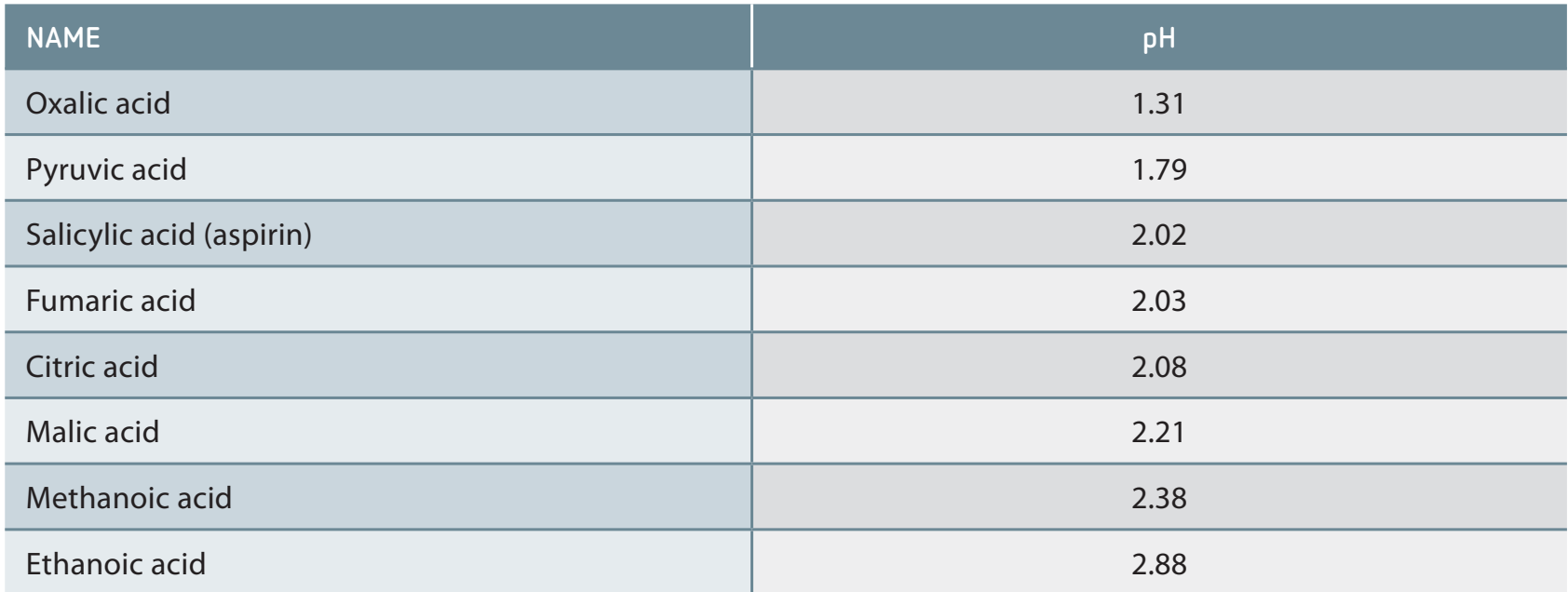
Examples of carboxylic acids:
- Formic acid: The simplest carboxylic acid, present in ant venom, and demonstrates high acidity.
- Benzoic acid: Found in plants, commonly used in preservatives due to its solid form at room temperature.
Remember: Utilise diagrams to visualise concepts such as acetic and formic acids, supporting diverse learning styles.
Overview
Carboxylic acids are vital in both nature and industry. Understanding their properties aids in predicting how they react in various situations. They are used in producing ethanoic acid (vinegar), a common household item.
Physical Properties
-
Boiling/Melting Points:
- Carboxylic acids have higher boiling and melting points than hydrocarbons and alcohols of comparable molecular weights.
- Hydrogen bonding leads to dimer formation, which significantly raises these temperatures.
- Example: Acetic acid (CH₃COOH, 118°C) compared to ethanol (C₂H₅OH, 78°C).
-
Solubility Factors:
- The carboxyl groups make these acids highly soluble in water.
- Effect of Chain Length:
- Short chains = highly soluble in water.
- Long chains = more soluble in organic solvents.
Chemical Properties
-
Acidity of Carboxylic Acids:
- Carboxylic acids donate protons (H⁺), classifying them as acids.
- Resonance stabilisation significantly influences their acidity, demonstrating the importance of structure.
- Example Reaction: When reacted with NaOH, carboxylic acids form soaps, showcasing the impact of their polarity.
-
Hydrogen Bonding and Polarity Effect:
- Hydrogen bonds play a substantial role in chemical reactions by enhancing interactions with both polar and non-polar compounds.
-
Substituents' Impact on Acidity:
- Electron-withdrawing groups (e.g., —NO₂) stabilise the carboxylate ion, thus increasing acidity.
- Electron-donating groups (e.g., —CH₃) decrease acidity by destabilising the carboxylate ion.
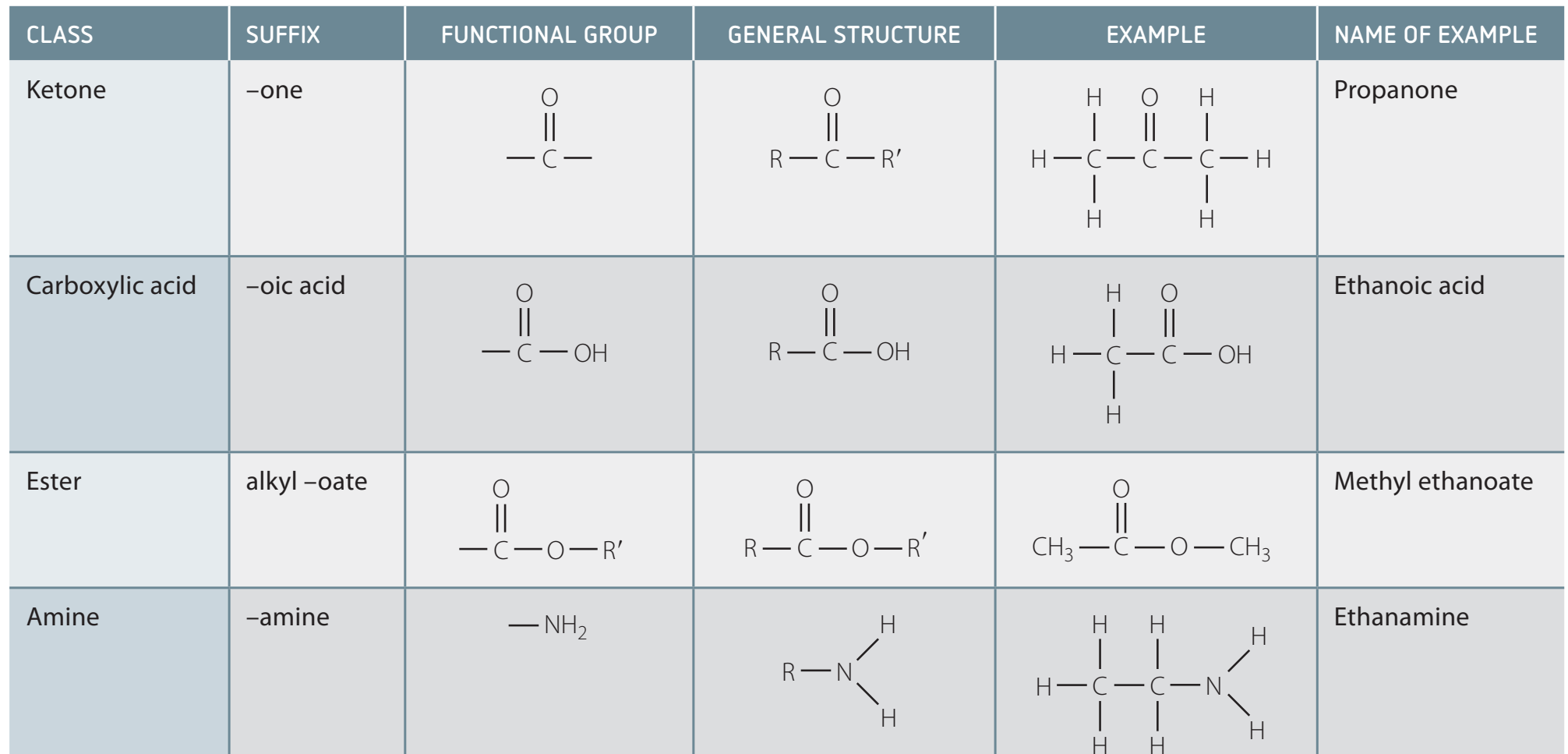
Comparison with Other Functional Groups
- Carboxylic acids are stronger due to their high acidity when compared to alcohols and ketones.
Synthesis and Reactions of Carboxylic Acids
Overview of Synthesis Methods
-
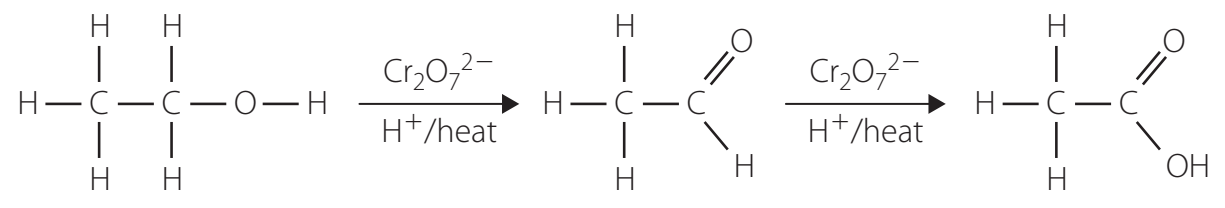
Oxidation of Alcohols and Aldehydes:
- Converts primary alcohols into carboxylic acids using potassium permanganate (KMnO₄).
- Conditions:
- Typically performed under basic pH conditions (around 8-10).
- Requires a heated, dilute aqueous solution.
- Explain Technical Terms:
- Reflux: A technique involving heating a chemical reaction mixture for an extended period while continually condensing the vapour back to liquid.
- Distillation: A process to purify or separate components of a liquid mixture by boiling and condensation.
Step-by-Step Breakdown:
- Step 1: Primary alcohol (R-CH₂OH) oxidised to aldehyde (R-CHO) using KMnO₄.
- Step 2: Aldehyde further oxidised to form carboxylic acid (R-COOH).
-
Hydrolysis of Nitriles:
- Converts nitriles into carboxylic acids via an amide intermediate.
- Example: The transformation of acetonitrile to acetic acid.
- Two-Stage Breakdown:
- Stage 1: Nitrile (R-CN) hydrolysed to form amide (R-CONH₂).
- Stage 2: Amide further hydrolysed to yield carboxylic acid (R-COOH).
Detailed Reactions of Carboxylic Acids
-
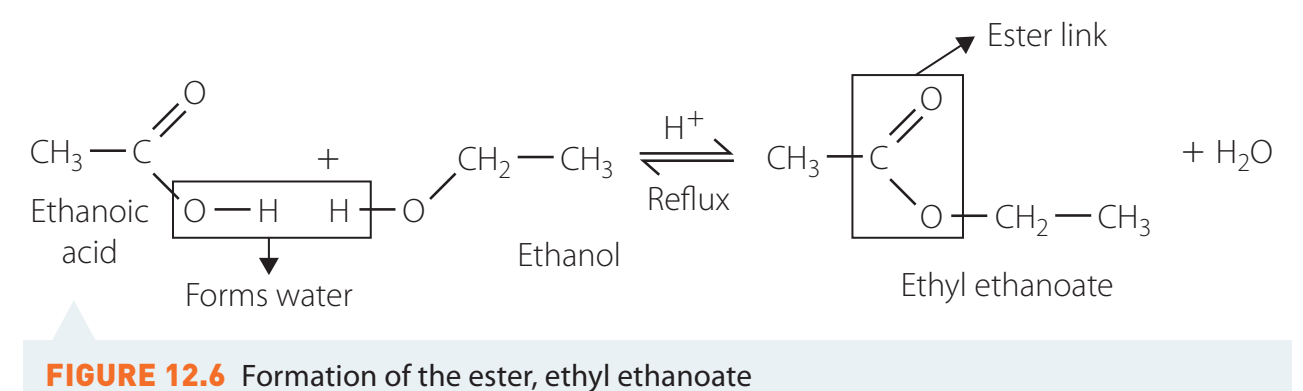
Esterification:
- Carboxylic acids react with alcohols in the presence of sulphuric acid (H₂SO₄) to form esters.
- Using Le Chatelier's Principle:
- Remove water to shift reaction equilibrium towards ester formation.
- Practical Example:
- Conversion of ethanoic acid and ethanol to form ethyl acetate.
- Hypothetical Setup:
- Mix reactants in the presence of H₂SO₄.
- Heat under reflux using a water bath.
- Gradually distil water to drive equilibrium to the right.
-
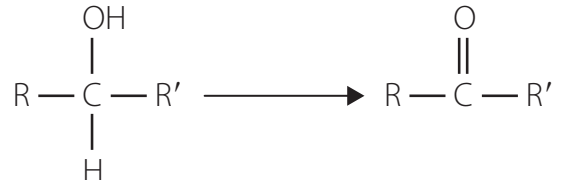
Reduction:
- Converts carboxylic acids to primary alcohols using lithium aluminium hydride (LiAlH₄).
- Contextual Application:
- Widely used in organic synthesis and the pharmaceutical industry.
- Involves reduction through intermediate aldehyde stages.
Important Mechanisms and Callouts
- Mechanistic Insight: Study mechanism flows and roles.
- Role of Catalysts: Catalysts increase rates without affecting equilibrium positions.
Functional Group Analysis and Influence on Reactivity
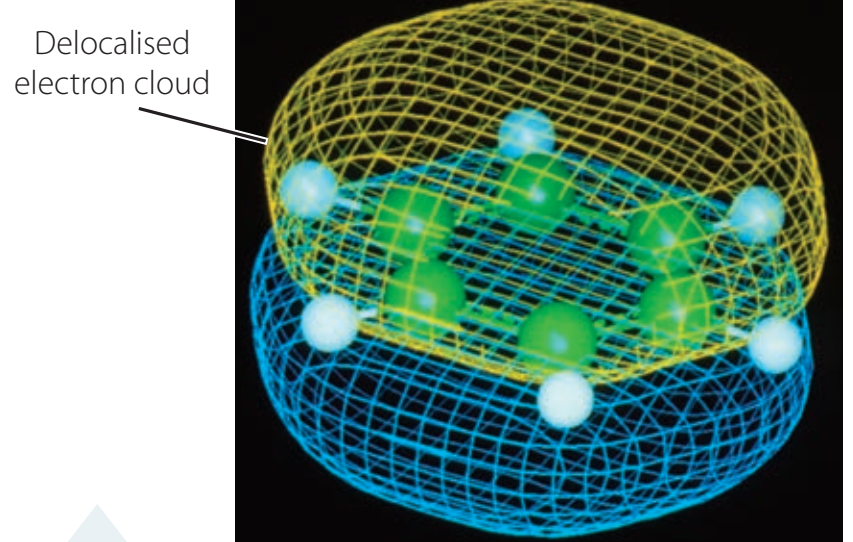
Structure and Resonance Stabilisation of the Carboxyl Group
- Understanding Resonance Through Diagrams:
- Resonance Spread: Delocalises negative charge across two oxygens.
- Resultant Stability: Stability is enhanced via the resonance effect.
- Pair diagram with essential features to reinforce key observations.
- Diagram: Clearly shows resonance structures.
Effects of Steric Hindrance
- Conceptual Introduction:
- Analogy: Similar to 'traffic congestion' for a relatable context.
- Steric Hindrance: Bulky groups obstruct reactivity.
- Example: Bulky groups, such as Tertiary butyl, act like roadblocks for nucleophiles.
- Visualises steric impacts in diagram representation.
- Diagram: Details steric hindrance effects.
Electronic Effects on Stability and Reactivity
- Inductive and Mesomeric Effects:
- Inductive Influence: Electron-withdrawing atoms adjust electron distribution.
- Mesomeric Adjustment: Through pi bond transfers.
- Provide a comprehensive example with a diagram.
- Diagram: Shows inductive effects with specificity.
Substituents and Functional Group Behaviour
- Substituent Impacts:
- Highlight chemical scenarios illustrating substituent effects on acidity.
- Introduce calculation models like the Hammett equation, with relevant examples.
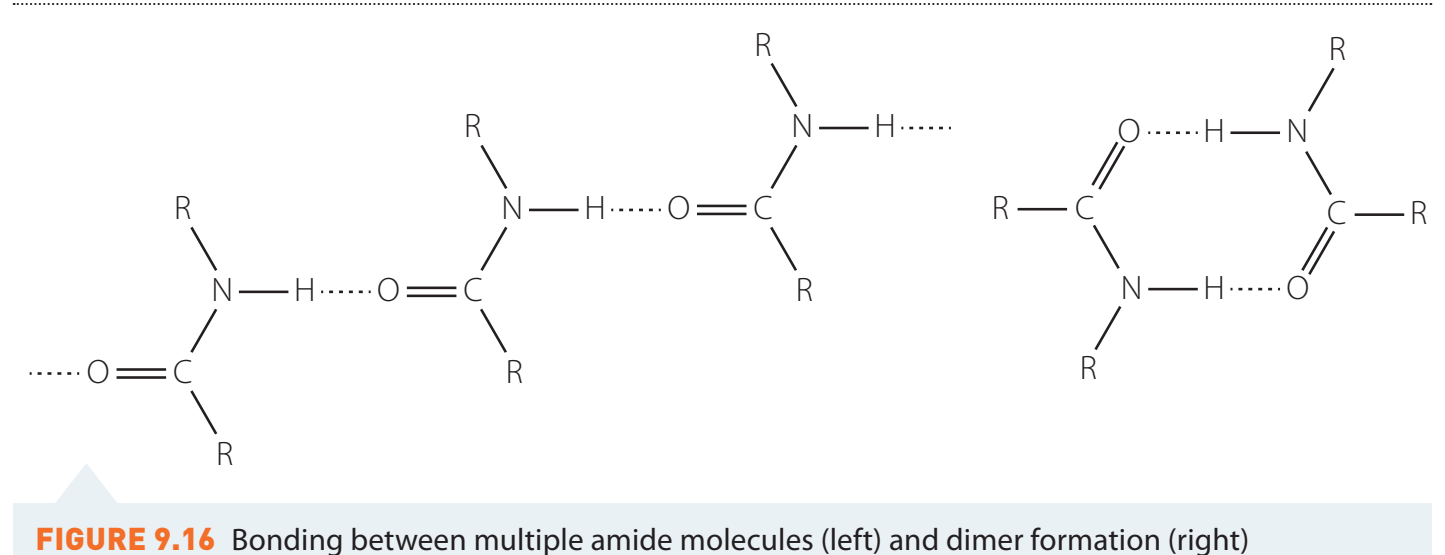
Intermolecular Forces Discussion
- Hydrogen Bonding Role:
- Carboxylic acids form dimers via hydrogen bonding. Increases boiling points compared to hydrocarbons.
- Consider impacts without hydrogen bonds, such as London dispersions.
- Diagram: Visual representation of hydrogen bonding dimers.
Reactivity Comparison with Other Functional Groups
- Comparative Insights:
- Explicitly compare acid reactivity versus aldehydes and ketones.
- Utilise examples and visual aids to demonstrate key differences and real-world implications.
- Post-Model Reflection: Prompt students to predict sequences showing competition or selectivity.
- Diagram: Table visualising reactivity differences.

Industrial Applications
Pharmaceutical Synthesis
- Carboxylic acids are crucial in producing medicines like aspirin and penicillin, essential for treating inflammation and infections.
- Key Roles:
- Improve drug solubility, aiding better drug delivery.
- Enhance effectiveness through ionisation, facilitating effective bodily interactions.
- Facilitating Drug Interactions:
- In cardiovascular drugs, they modulate bioavailability.
- Aid in synthesising antibiotics by enhancing binding affinities.
Food Industry Roles
-
Acetic Acid (Vinegar):
- Utilised in salad dressings.
- Enhances flavour in pickles and sauces.
-
Citric Acid:
- Found in sour candies and sodas.
- Regulates pH and enhances flavour with tartness.
Environmental and Sustainability Considerations
Efforts are underway to reduce environmental impact using biodegradable acids and renewable sources.
- Key companies are leading sustainable practices.
- Successful Impact Assessments: Demonstrated tangible environmental benefits facilitating large-scale implementations.
Environmental impact assessments are essential for ensuring sustainable practices.
Role in Ester Production
Flavours and Fragrances Industry
- Relevance: Esters derived from carboxylic acids create appealing scents and flavours key for product success.
- Simplified Definition:
- Esters form through the reaction between carboxylic acids and alcohols.
- They enhance product appeal through desired aromas and tastes found in perfumes and foods.

Interaction and Activity
Interactive Exercises
-
Activity 1: Complete a written task or discussion on evaluating a carboxylic acid's environmental impact, with clearly defined outcomes.
-
Activity 2: Investigate and assess sustainability practices in products containing carboxylic acids.
-
Quiz Ideas:
- True/False questions with detailed answers for comprehensive understanding.
- Interactive matching exercises accessible online for practice.
Visual Materials
- Explanations: Each diagram includes annotations explaining key points.
- Enhancing Critical Thinking: Diagrams include questions to encourage deeper examination.
Practice Problems and Worked Examples
Introduction to Practice Problems
Consistent practice is key for mastering carboxylic acids. Focus on identifying structures, predicting reactions, and performing calculations.
Identifying Carboxylic Acids
- Problem Sets: Identify the —COOH group in complex molecules.
- Recognise this functional group's position and role in larger structures.
Focus sharply on the —COOH group. Its identification is critical and often a key component of recognising carboxylic acids.

Reaction Equations
- Exercises:
- Write and balance reaction equations.
- Include key specifics like catalysts and temperatures.
- Steps:
- Identify reactants and products.
- Formulate the reaction equation.
- Balance, considering all reactants, products, and conditions.
Pay attention to catalysis and temperature conditions. These often significantly impact the reaction pathways and results.
Calculating pH of Carboxylic Acid Solutions
- Example Problem:
- Calculate pH using weak acid properties and the acid dissociation constant, .
- Solve:
- Dissociation Equation:
- Equilibrium Expression:
- Calculate: Assume and solve based on initial concentrations.
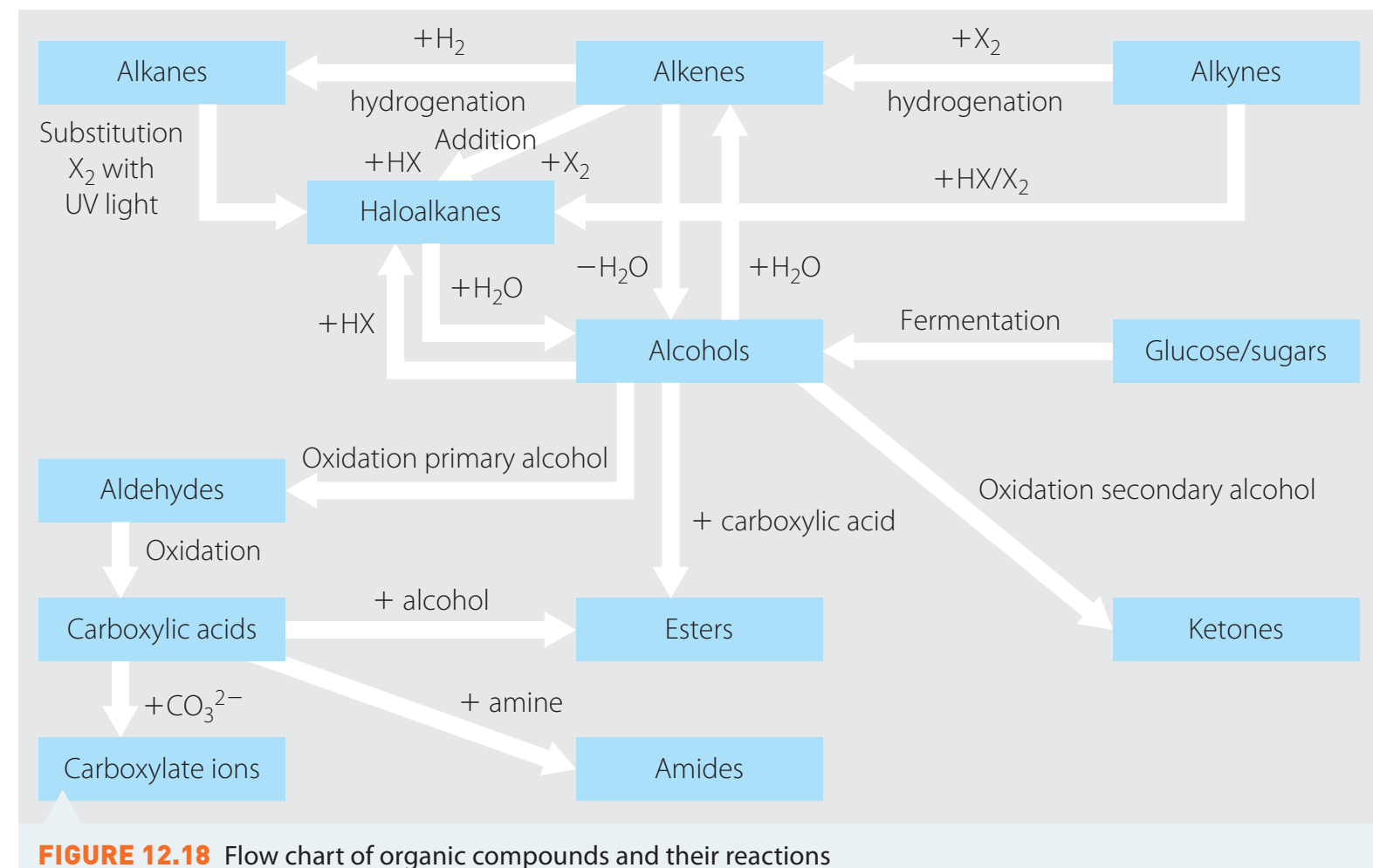
Organic Synthesis Problems
- Worked Examples:
- Convert alcohols to and from carboxylic acids.
- Focus on key synthesis steps illustrated in the flowchart.
Understand and identify key stages in synthesis. This is crucial for effective conversion.

Illustrative Mechanisms
- Mechanism Snapshots:
- Focus on transformation mechanisms like esterification.
- Visuals Emphasise:
- Transition states.
- Intermediates.
Visualise transition states and intermediates for a thorough understanding of reaction mechanics.

Critical Thinking Exercises
- Exercises:
- Predict reaction outcomes based on structural modifications.
- Consider steric and electronic effects on reactivity.
Analysing Reaction Conditions
- Interactive Questions:
- Explore varying effects of temperature, pressure, and catalysts on reactions.
- Apply understanding to real scenarios.
Summary
- Reinforce that consistent practice is essential for mastering the theories and applications of carboxylic acids.
- Encourage self-assessment and the use of additional resources to further learning.

500K+ Students Use These Powerful Tools to Master Carboxylic Acids For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
299 flashcards
Flashcards on Carboxylic Acids
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Carboxylic Acids
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Carboxylic Acids
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Carboxylic Acids
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Carboxylic Acids
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Carboxylic Acids you should explore
Discover More Revision Notes Related to Carboxylic Acids to Deepen Your Understanding and Improve Your Mastery
