Photo AI
Last Updated Sep 24, 2025
Amides - Functional Group Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Amides - Functional Group quickly and effectively.
443+ students studying
Amides - Functional Group
What are Amides?
Amides are organic compounds characterised by a carbonyl group (C=O) connected to a nitrogen atom (N).
Carbonyl Group: Comprises a carbon atom double-bonded to an oxygen atom.
Differentiation
- Amines vs Amides:
- Amines: Do not contain a carbonyl group.
- Amides: Include a carbonyl group.
- Esters vs Amides:
- Esters: Contain an oxygen atom bonded to the carbonyl group.
- Amides: Feature a nitrogen atom bonded to the carbonyl group.
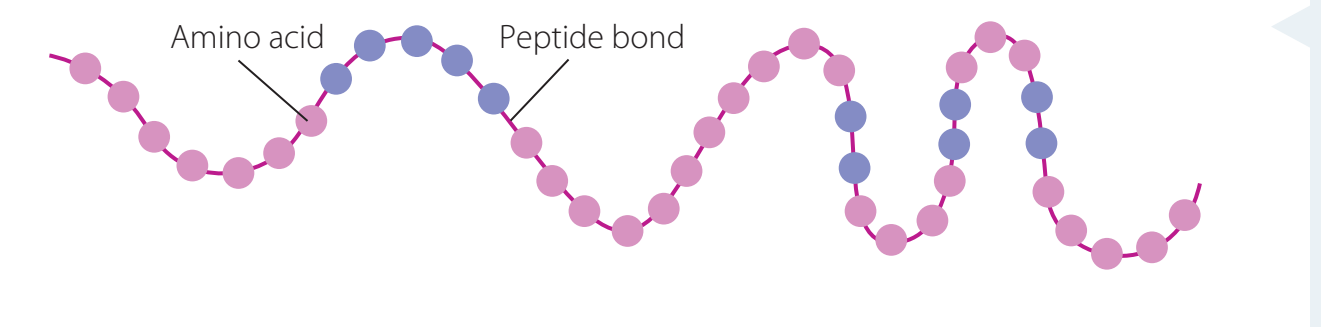
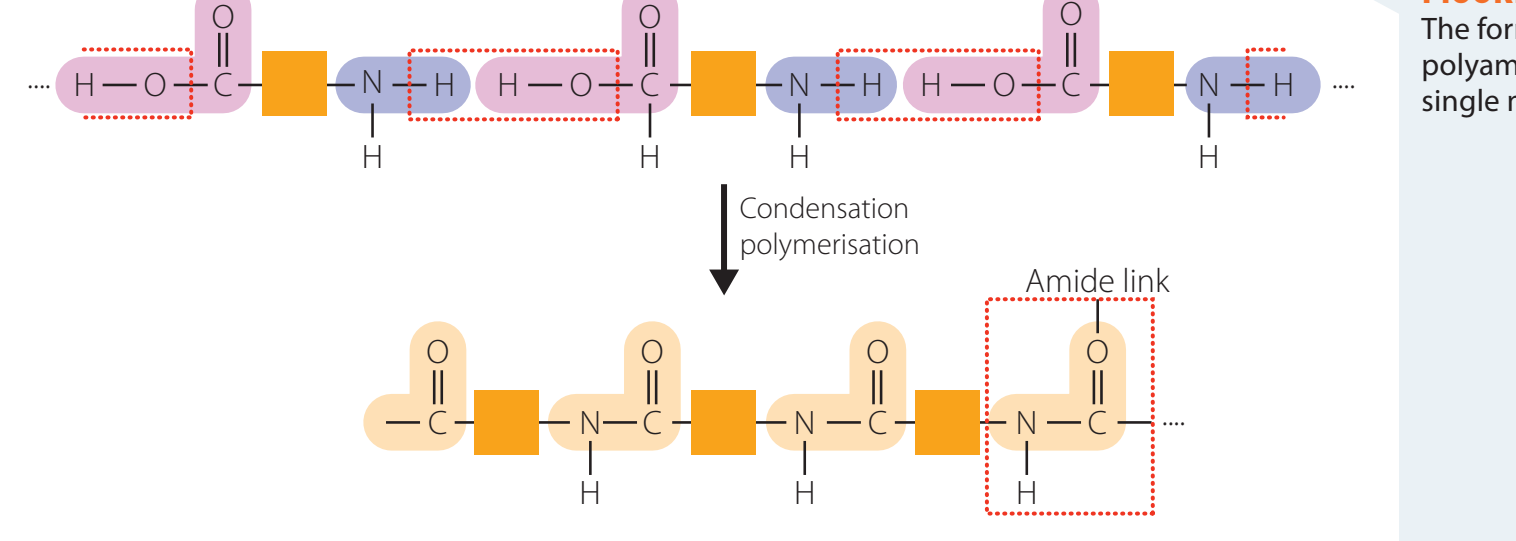
Role in Biological Systems: Amides form peptide bonds, which are crucial connections linking amino acids in proteins.
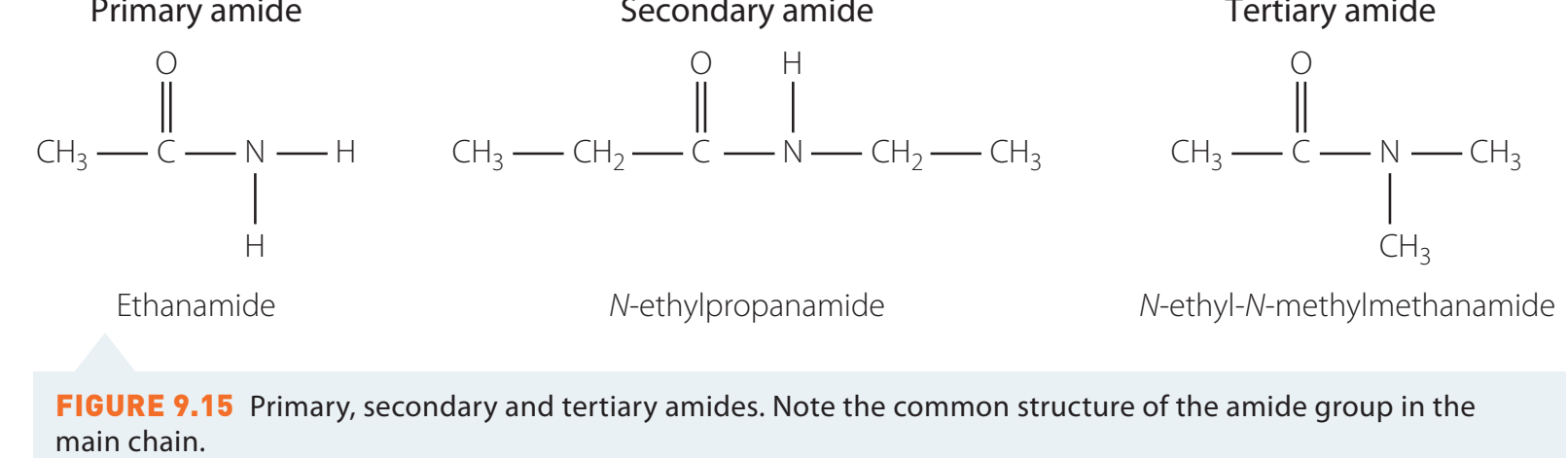
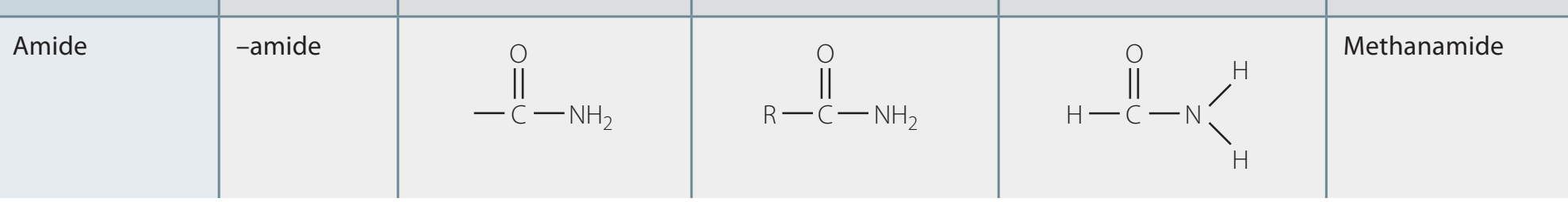
Structural Formula
The general structural representation of amides is R-CO-NR'R''.
-
Example Table:
Compound Structural Formula Acetamide CH₃CONH₂ Formamide HCONH₂
Nomenclature Basics
Naming Conventions
- Derived from carboxylic acids and alkyl groups.
- Example: Acetic Acid gives rise to Acetamide.
- Substituted Amides:
- Example: N-methylacetamide denotes a methyl group attached to nitrogen.
Example Table:
| Base Acid | Amide Name |
|---|---|
| Acetic Acid | Acetamide |
| Formic Acid | Formamide |
Role in Peptide Bonds
Peptide Bonds: Fundamental connections forming the proteins' primary structure.
- Peptide bonds incorporate amide linkages, essential for maintaining protein structure.
IR Spectroscopy and Amides
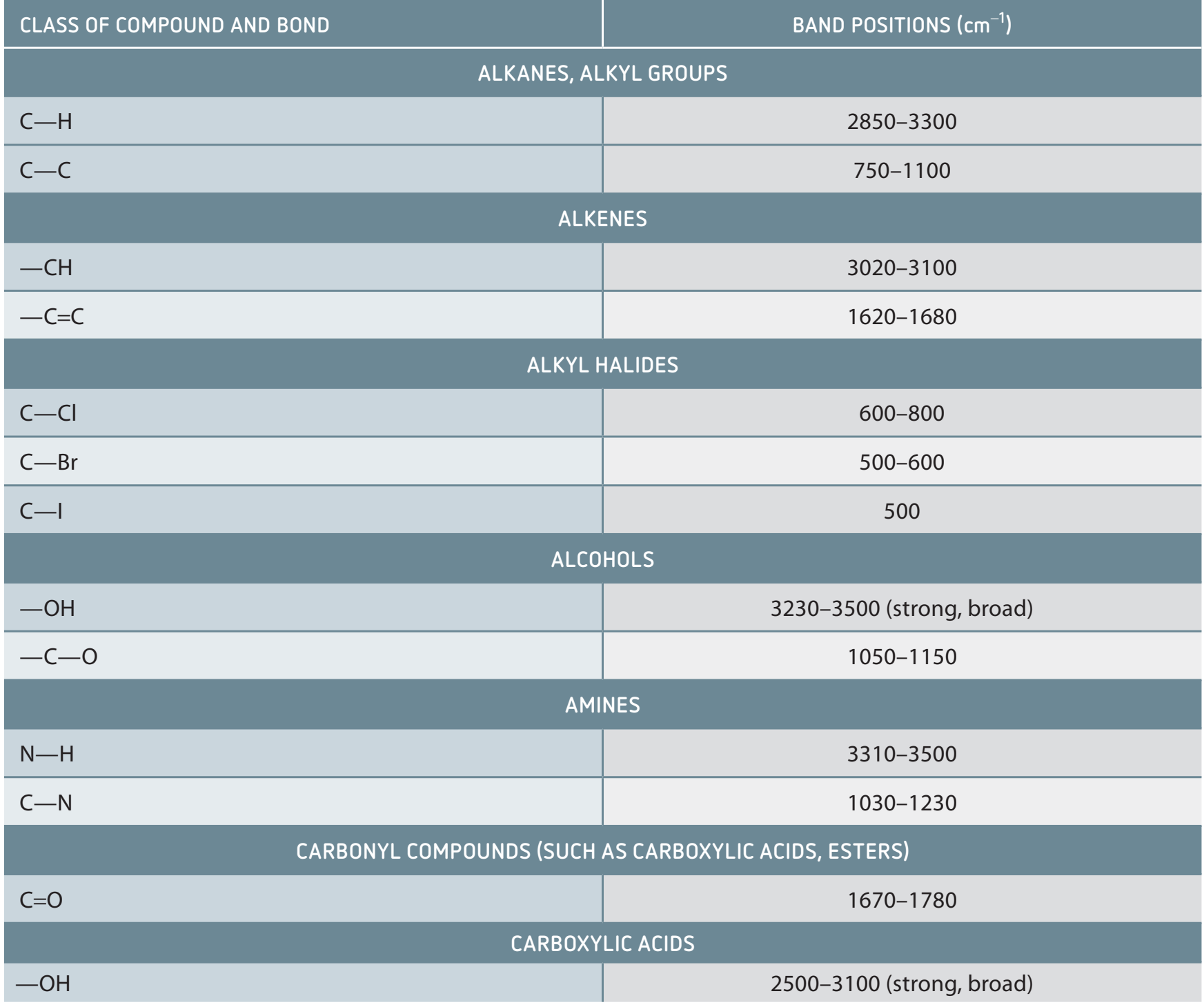
Infrared (IR) Spectroscopy aids in identifying functional groups in molecules by analysing their absorption of infrared light. Amides possess distinctive IR characteristics, important in the study of pharmaceuticals and proteins.
IR Spectroscopy Importance: Provides insights into molecular structures, instrumental in identifying substances like amides.
Key Concepts of IR Spectroscopy for Amides
- Technique Explanation:
- Measures vibrational energy absorption to identify structural components.
- Crucial for distinguishing amide groups in biological samples.
IR Spectral Peaks for Amides
-
Characteristic Peaks:
- C=O Stretching: Found between 1630-1690 cm.
- NH Stretching: Occurs between 3300-3500 cm.
Real-World Application: IR spectroscopy's capability to identify amide functional groups is crucial for drug development and understanding biological systems.
Variation in Amides
- Primary Amides: Contain two N-H bonds.
- Secondary Amides: Feature one N-H bond.
- Tertiary Amides: Do not have N-H bonds.
Chemical Properties Overview
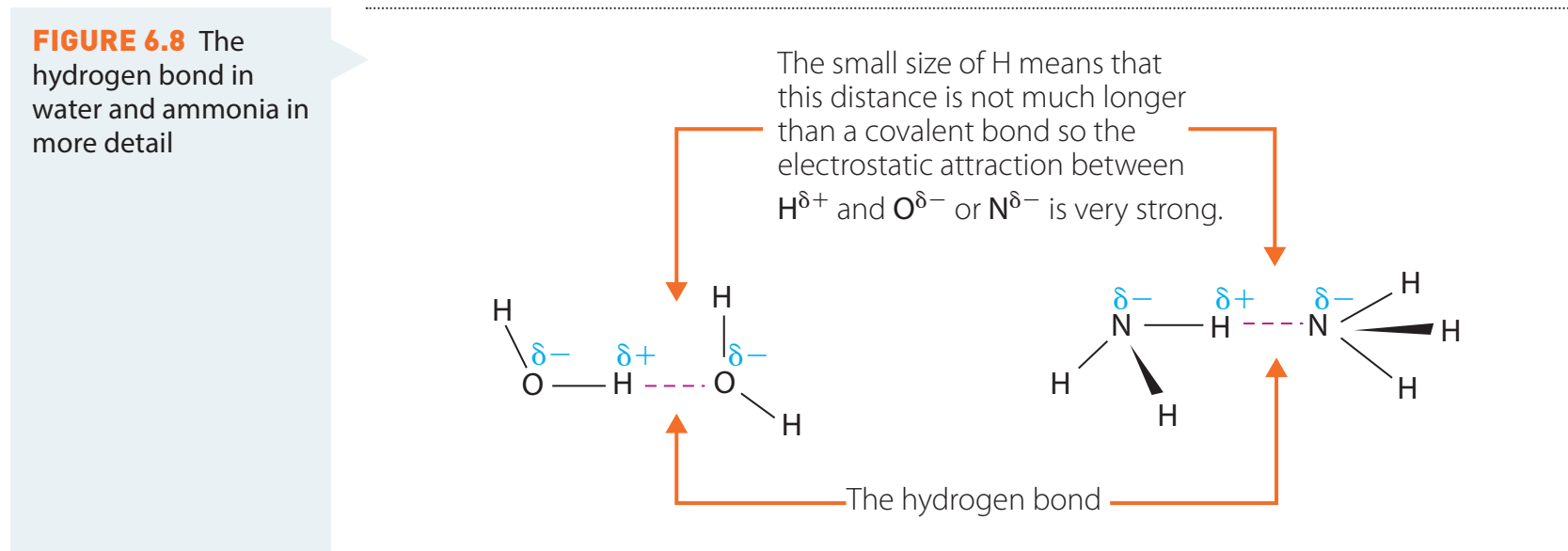
- Basicity and Acidity Analysis: Amides are weaker bases relative to amines due to the resonance of the lone pair on nitrogen with the carbonyl group, limiting its availability for protonation.

- Stability and Resonance: Resonance in amides results in enhanced stability compared to other carbonyl compounds.
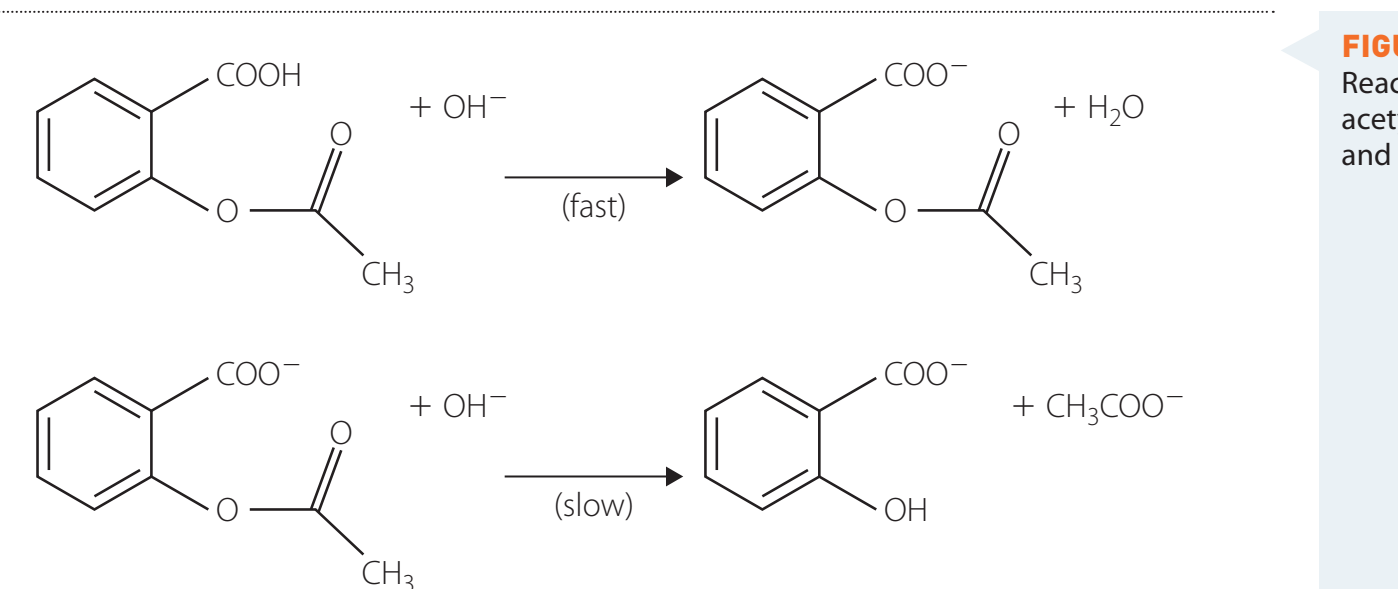
Common Reactions of Amides
-
Hydrolysis Reactions:
-
Undergo acidic and basic hydrolysis.
- Acid-Catalysed: Leads to protonation and formation of carboxylic acids.
- Base-Catalysed: Results in carboxylate ions.
-
-
Reduction Reactions:
- Use of LiAlH₄ results in reduction to amines:
-
Comparison Table: Reaction Type vs. Observations:
Reaction Type Example Key Observations Hydrolysis Acidic and basic Differences in rate Reduction Using LiAlH₄ To amines
Synthesis of Amides
- Overview: Amides can be synthesised by reacting carboxylic acids with amines in the presence of an acid catalyst.
- Example Reaction: Carboxylic Acid + Amine → Amide (with Acid Catalyst)
Synthesis Using Acid Chlorides
- Detailed Steps:
- Step 1: Acid chloride reacts with an amine.
- Step 2: Formation of an intermediate complex.
- Step 3: Completion to form the amide.
Understanding the application of catalysts can significantly influence reaction outcomes.
Industrial Applications
Amides play a vital role in the synthesis of polymers like nylon, which are important across various industrial sectors.
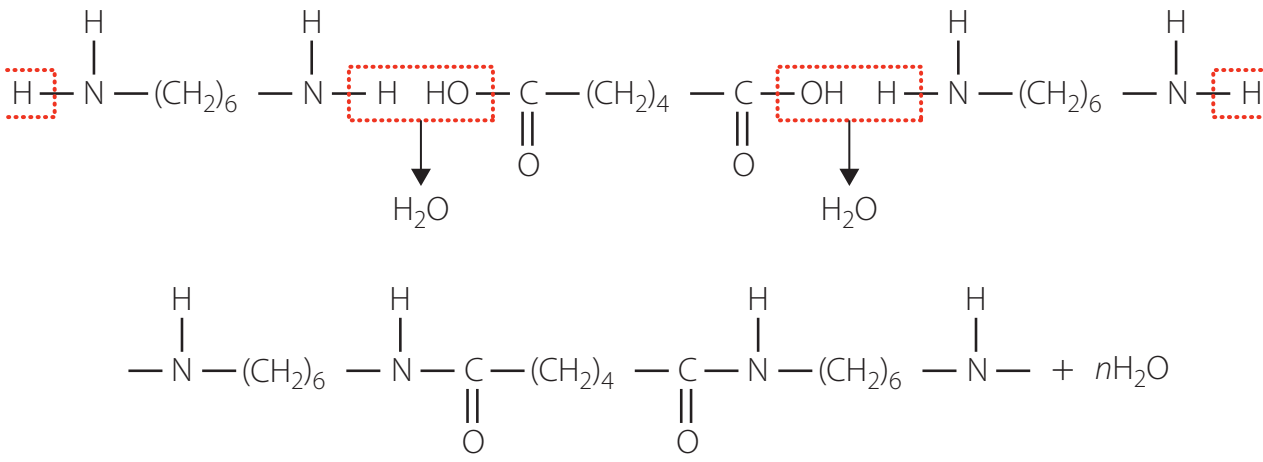
Practice Synthesis Questions
Question 1: What product would be formed from the reaction of benzoyl chloride with methylamine? Solution: N-methylbenzamide would be formed as the methyl group from methylamine attaches to the nitrogen, and the carbonyl group from benzoyl chloride forms the amide linkage.
Question 2: Explain how to convert butanoic acid to butanamide. Solution: First, convert butanoic acid to butanoyl chloride using SOCl₂. Then react butanoyl chloride with ammonia to form butanamide.
Question 3: Predict the product when ethanamide undergoes hydrolysis in acidic conditions. Solution: Ethanamide would hydrolyse to form ethanoic acid and ammonium ions.
Amides in Biological Systems
Introduction to Amides in Biological Systems
Amides: Represent a functional group with a carbonyl group (C=O) linked to a nitrogen atom (N).
- Role: Amides connect amino acids to form proteins via peptide bonds.
- Essential in biochemistry.
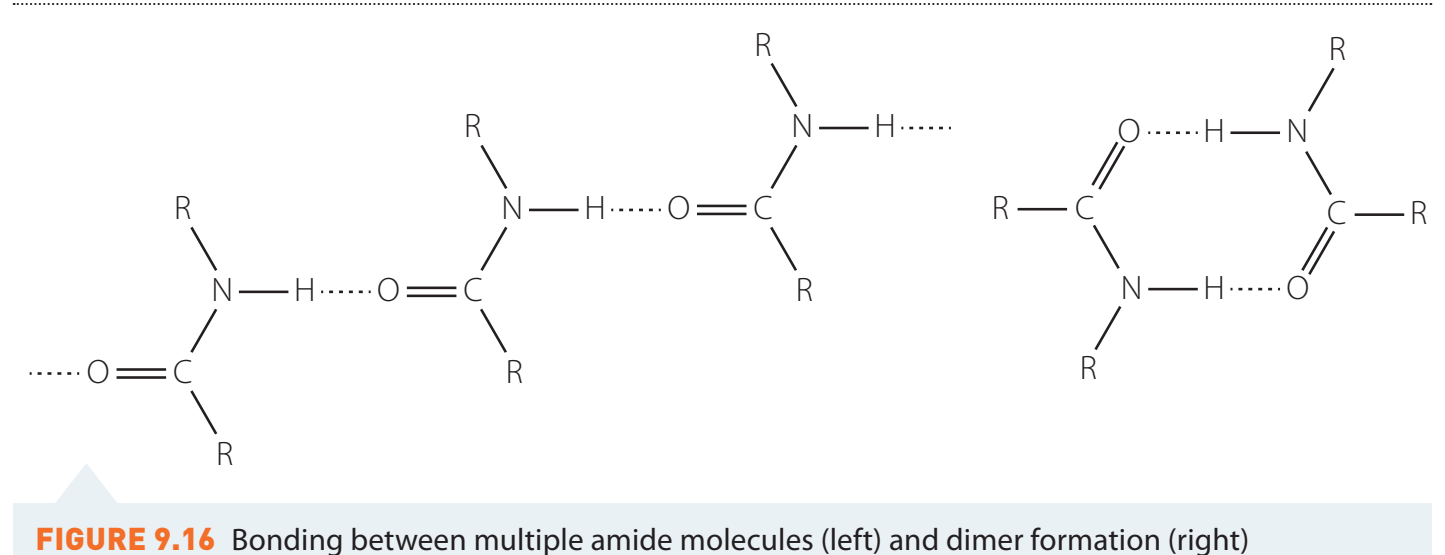
Influence on Protein Folding and Stability
- Amide linkages assist in ensuring protein stability.
- Supports structures like alpha-helices and beta-sheets.
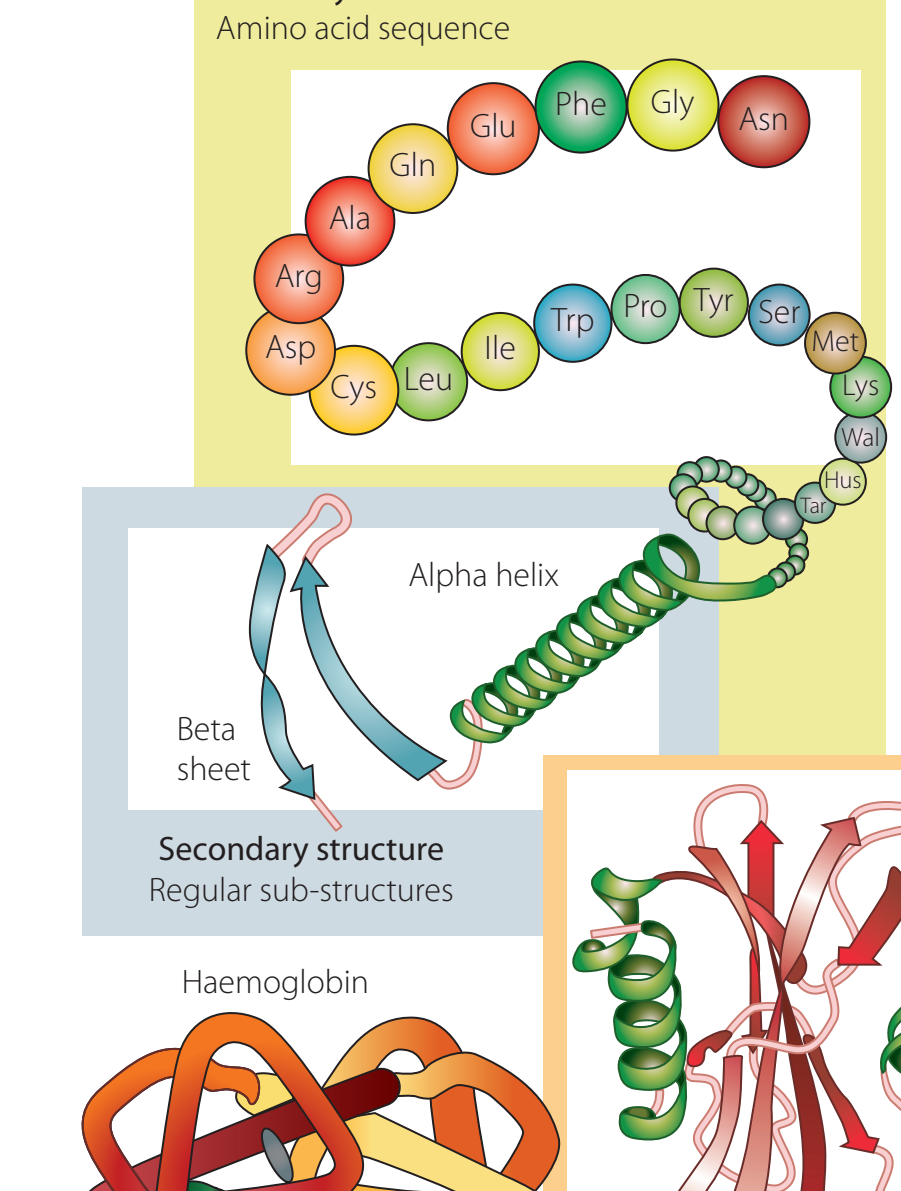
Examples of Amides in Proteins
-
Haemoglobin
- Amide bonds uphold its quaternary structure.
Amides and Environmental Impacts
- Biodegradability and Ecosystem Interaction:
- Determines natural decomposition of amides. Extended presence can impose negative effects on aquatic and terrestrial environments.
-
Pollution Sources:
- Industrial and agricultural practices are major contributors.
-
Environmental Consequences:
- Potential to cause algal blooms and diminished soil fertility.
Mitigation Strategies
- Effective industrial waste management.
- Employ phytoremediation to absorb and decompose amides.
500K+ Students Use These Powerful Tools to Master Amides - Functional Group For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
299 flashcards
Flashcards on Amides - Functional Group
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Amides - Functional Group
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Amides - Functional Group
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Amides - Functional Group
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Amides - Functional Group
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Amides - Functional Group you should explore
Discover More Revision Notes Related to Amides - Functional Group to Deepen Your Understanding and Improve Your Mastery
