Photo AI
Last Updated Sep 24, 2025
Boyle's Law Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Boyle's Law quickly and effectively.
350+ students studying
Boyle's Law
Introduction to Gas Laws
- Gas Laws: Essential concepts that describe how gases behave in relation to pressure, volume, and temperature.
- Importance: Vital for understanding gas dynamics in diverse contexts, including atmospheric phenomena, industrial processes, and common applications such as airbags.
Boyle's Law Defined
Boyle's Law: At a constant temperature, pressure is inversely proportional to volume. It is mathematically represented as .
- Example Representation: When volume is halved, pressure is doubled.
- Real-world Example: Consider compressing a balloon or gas in a piston of an engine.
Core Principles of Boyle's Law
-
Inverse Relationship: As pressure increases, volume decreases proportionally, ensuring the product remains constant.
-
Mathematical Expressions: The equation can be used to compare different states of a gas, assuming temperature is constant.
Maintaining a constant temperature is essential.
Real gases may behave differently at high pressures or low temperatures. In these instances, the Van der Waals equation provides more accurate results.
Historical Context and Development
- Robert Boyle: Identified the inverse relationship between pressure and volume through experiments with a J-tube in the 17th century.
- Significance in Scientific Methodologies: His precision with air pumps initiated a new era of scientific accuracy.
Boyle's work in collaboration with the Royal Society was pivotal in establishing the law's recognition and scientific validity.
Integration with Ideal Gas Law
- Ideal Gas Law () showcases Boyle's Law as a particular scenario where temperature remains constant: .
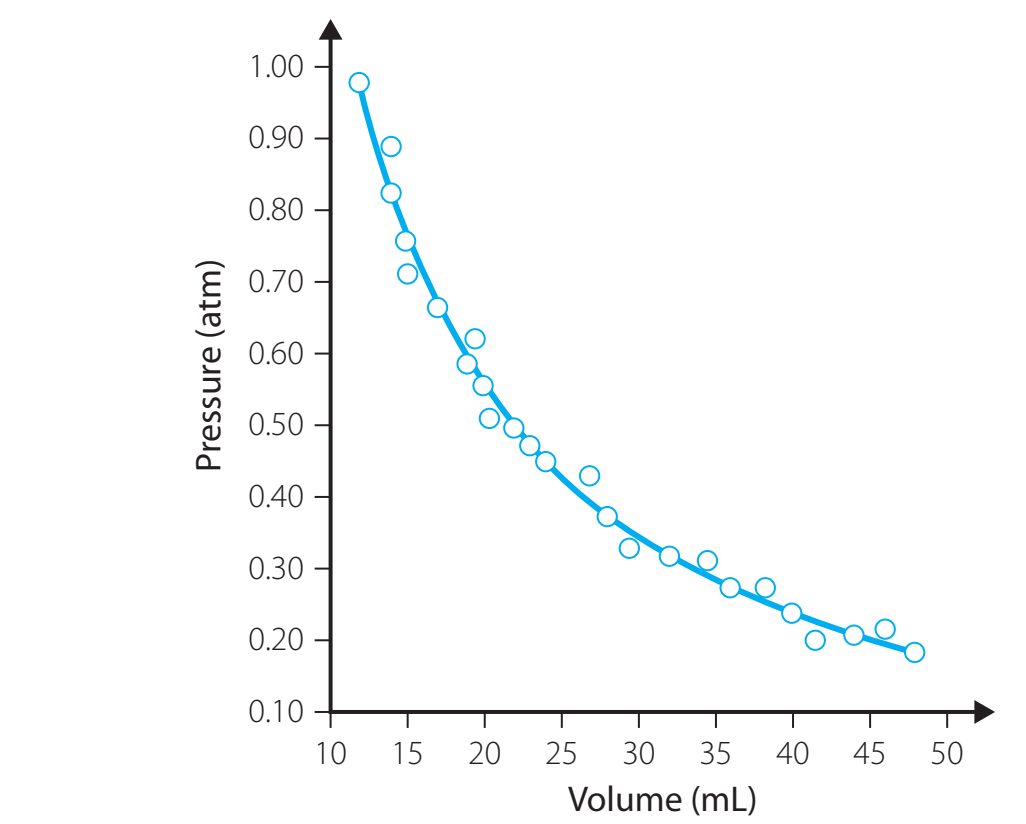
Graphical Representation
- Hyperbolic Curve: Illustrates the inverse relationship between pressure and volume.
Practical Application & Experimentation
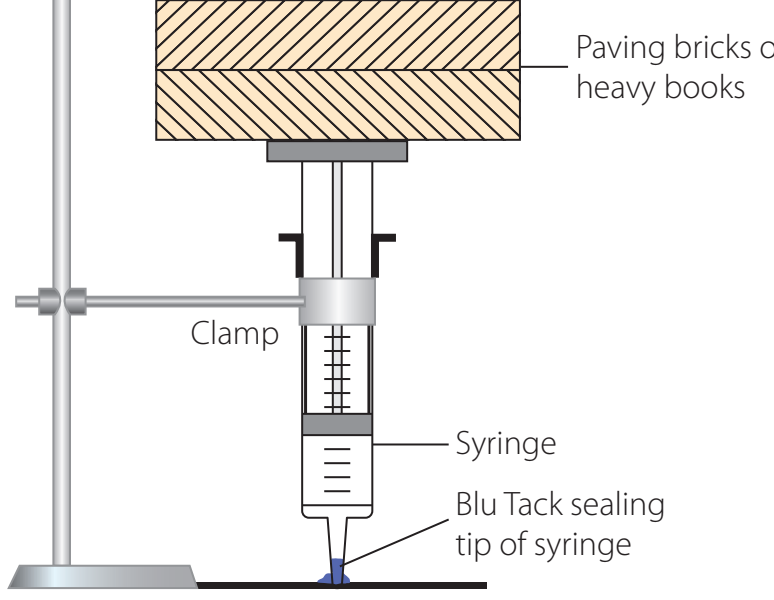
Experiment Setup
- Materials: Syringes, pressure sensors, data logger, sealed container.
- Procedure: Record the changes in pressure corresponding to volume adjustments using syringes and document the data through sensors.
Mathematical Manipulation and Problem-Solving
- Rearrangement: or .
Worked Examples
-
Simple Calculation: If a gas has an initial pressure of 2 atm and volume of 4 L, what will the pressure be if the volume is reduced to 2 L?
Given:
Using Boyle's Law:
Rearranging to find :
Therefore, the new pressure is 4 atmospheres.
-
Intermediate Example: A gas sample has a pressure of 101.3 kPa and occupies 2000 cm³. Calculate the pressure when compressed to 1000 cm³ at constant temperature.
Given:
Using Boyle's Law:
Rearranging to find :
Therefore, the new pressure is 202.6 kPa.
Exam Tips
- Consistently use the correct units.
- Use mnemonics, such as "TV needs PRatchet," to remember temperature-pressure-volume relationships.
A mastery of these concepts enables students to confidently tackle science exams and effectively address real-world issues like environmental changes or engineering problems.
500K+ Students Use These Powerful Tools to Master Boyle's Law For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
136 flashcards
Flashcards on Boyle's Law
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Boyle's Law
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes10 questions
Exam questions on Boyle's Law
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Boyle's Law
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Boyle's Law
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Boyle's Law you should explore
Discover More Revision Notes Related to Boyle's Law to Deepen Your Understanding and Improve Your Mastery
