Photo AI
Last Updated Sep 24, 2025
Gas Laws: Avogadro's Law Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Gas Laws: Avogadro's Law quickly and effectively.
423+ students studying
Gas Laws: Avogadro's Law
Introduction
Avogadro's Law: At a constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas.
Avogadro's Law: Under constant temperature and pressure conditions, the volume of a gas directly correlates with the number of moles of the gas.
Historical Context:
- 1811: Amedeo Avogadro introduced his hypothesis that equal volumes of gases, at identical conditions, contain an equal number of molecules.
- 1860 Karlsruhe Congress: Avogadro's hypothesis gained recognition through advanced experimental techniques.
Mathematical Representation
- Formula: The relationship between volume and moles is given by or .
- is the volume of the gas, is the number of moles, and is the proportionality constant.
Example Calculation:
- For 2 moles of gas at STP with , the volume is calculated as .
Experimental Validation
Significance: Experiments substantiate Avogadro's Law in practical scenarios.
Historical Experiments:
- Joseph Louis Gay-Lussac's Experiments: Explored fixed volume ratios in chemical reactions, which influenced Avogadro's hypothesis.
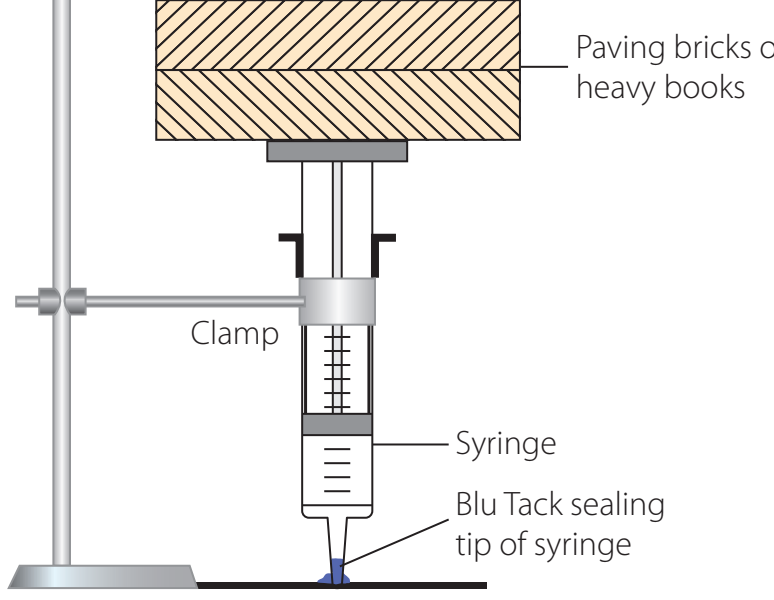
Methodology:
- Setup: Employ a gas syringe under controlled parameters.
- Process: Observe gas volumes under stable conditions.
EXPECTED OUTCOMES: Observations should confirm the volume-mole proportionality.
Graphical Analysis
- Graph Construction:
- Represent volume (V) on the y-axis and moles (n) on the x-axis.
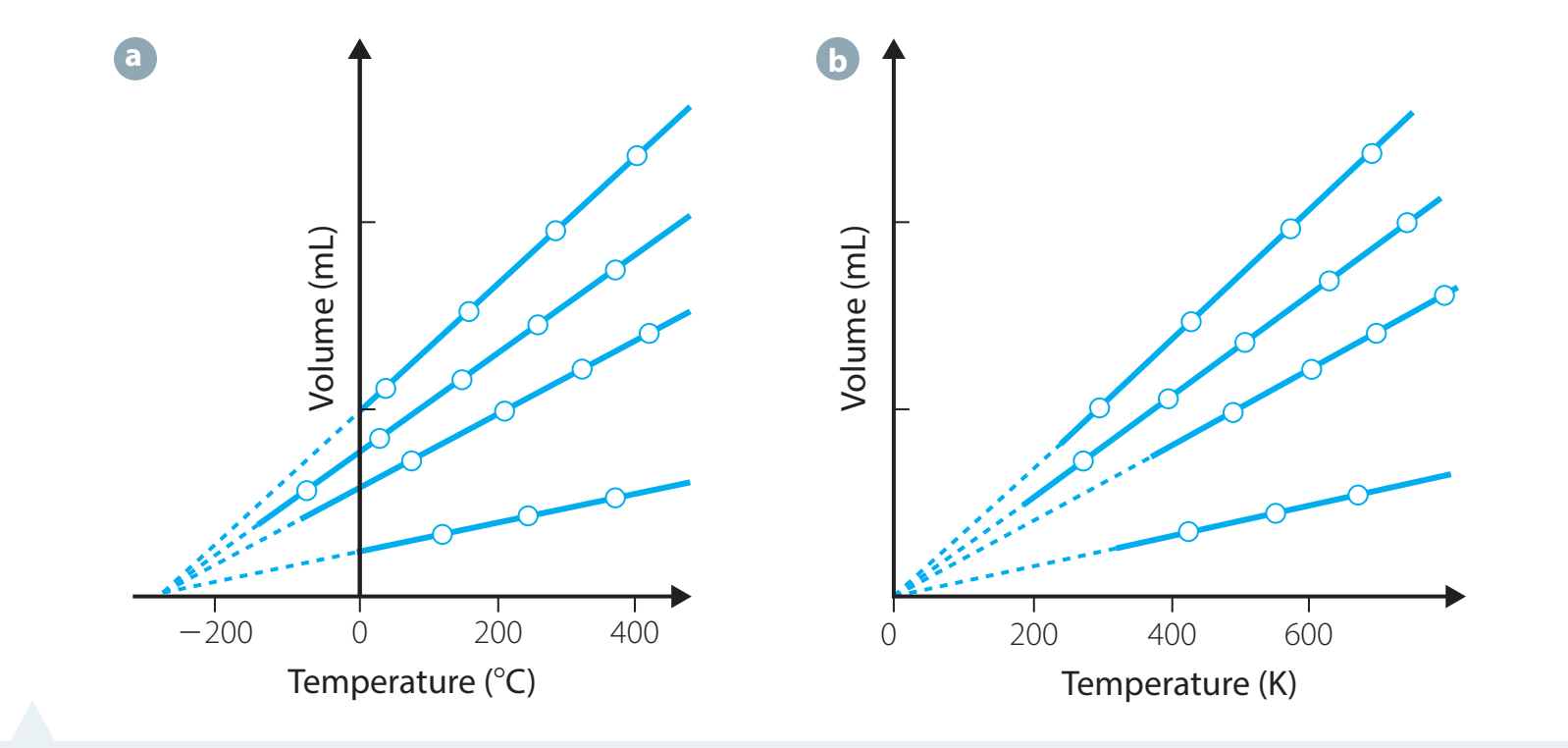
- Interpretation: A linear graph signifies direct proportionality, consistent with Avogadro's Law.
Integration with Other Laws
Avogadro's Law is part of the Ideal Gas Law: , corresponding with:
- Boyle's Law: Inverse relationship between pressure and volume
- Charles's Law: Direct relationship between volume and temperature
- Gay-Lussac's Law: Direct relationship between pressure and temperature
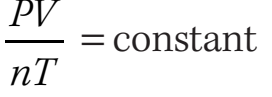
Common Misconceptions
-
Misbelief in Molar Volume Variation:
- Reality: At STP, molar volume remains constant for different gases.
-
Confusion with Mass:
- Greater molar mass does not imply a larger volume at STP.

Exam Tip: Ensure conditions are constant. Identify deviations from STP, which can lead to non-ideal behaviour.
Practice Problems
-
Problem: Calculate the volume of 4 moles at STP.
- Solution:
-
Problem: At non-STP conditions, with 5 moles and a volume of 120 L, determine .
- Solution:
By mastering Avogadro's Law, students can better grasp gas behaviour, essential for both examinations and practical applications in science and industry.
500K+ Students Use These Powerful Tools to Master Gas Laws: Avogadro's Law For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
136 flashcards
Flashcards on Gas Laws: Avogadro's Law
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Gas Laws: Avogadro's Law
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes10 questions
Exam questions on Gas Laws: Avogadro's Law
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Gas Laws: Avogadro's Law
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Gas Laws: Avogadro's Law
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Gas Laws: Avogadro's Law you should explore
Discover More Revision Notes Related to Gas Laws: Avogadro's Law to Deepen Your Understanding and Improve Your Mastery
