Photo AI
Last Updated Sep 24, 2025
Gas Properties and Laws Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Gas Properties and Laws quickly and effectively.
424+ students studying
Gas Properties and Laws
Exploring the behaviour of gases is fundamental for understanding essential scientific and practical principles. By examining key properties and laws such as pressure, volume, and temperature, we can more precisely explain phenomena like inflating balloons and atmospheric pressures.
Properties of Gases
Definition of Key Properties
-
Pressure (P):
infoNotePressure: The force exerted per unit area by gas molecules against the walls of their container. Common units include atmospheres (atm), pascals (Pa), and kilopascals (kPa).
- Pressure results from molecular collisions with container walls.
- 1 atm is equivalent to 101.3 kPa.
- Example: Heating a sealed balloon increases molecular movement, thereby increasing pressure.
-
Volume (V):
infoNoteVolume: The amount of space that a gas occupies. Standard units are litres (L) and millilitres (mL).
- Volume is dependent on the container's size.
- Example: Compressing gas in a syringe reduces volume as the available space decreases.
-
Temperature (T):
infoNoteTemperature: A measure of the average kinetic energy of gas particles. Use the Kelvin (K) scale to prevent negative values.
- The Kelvin scale is essential for calculations involving gas laws.
- Example: Converting 25°C to Kelvin:
-
Amount of Gas (n):
infoNoteAmount of Gas: Measured in moles using Avogadro's number ( particles per mole).
- Scenario: One mole of oxygen gas in a 22.4-litre container at standard temperature and pressure (STP).
Table of Gas Property Units
| Property | Common Units |
|---|---|
| Pressure | atm, Pa, kPa |
| Volume | L, mL |
| Temperature | K |
| Amount of Gas | mol |
Call-outs and Key Concepts
-
Pressure Conversion Note: Use 1 atm = 760 mmHg = 101.3 kPa for conversions.
-
Example Highlight: A balloon's pressure changes with temperature, described by the ideal gas law.
Diagrams with Captions
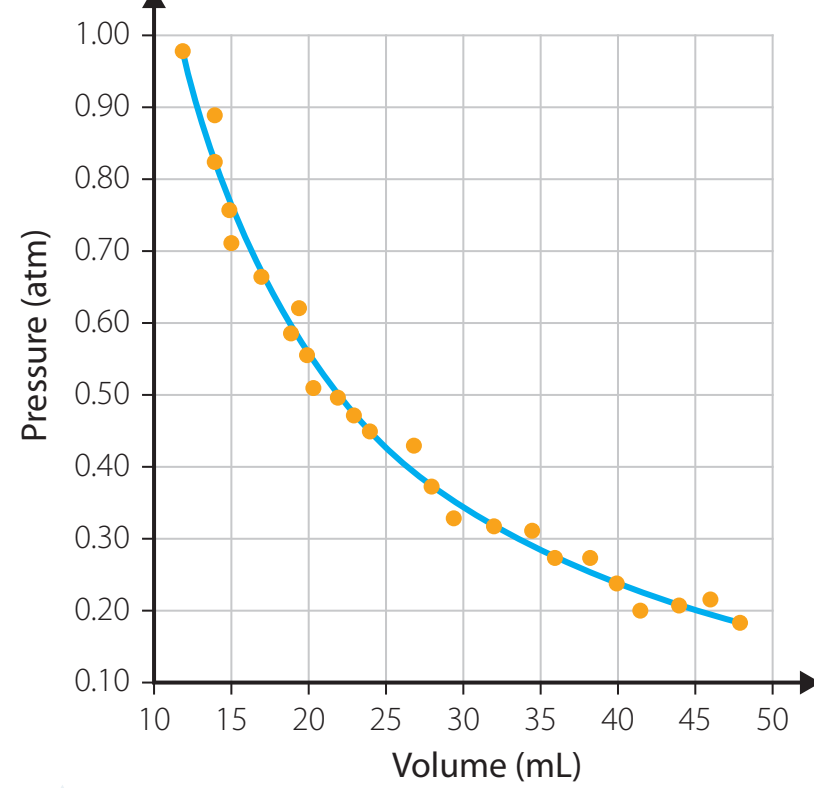
- Caption: The inverse relationship between volume (V) and pressure (P).
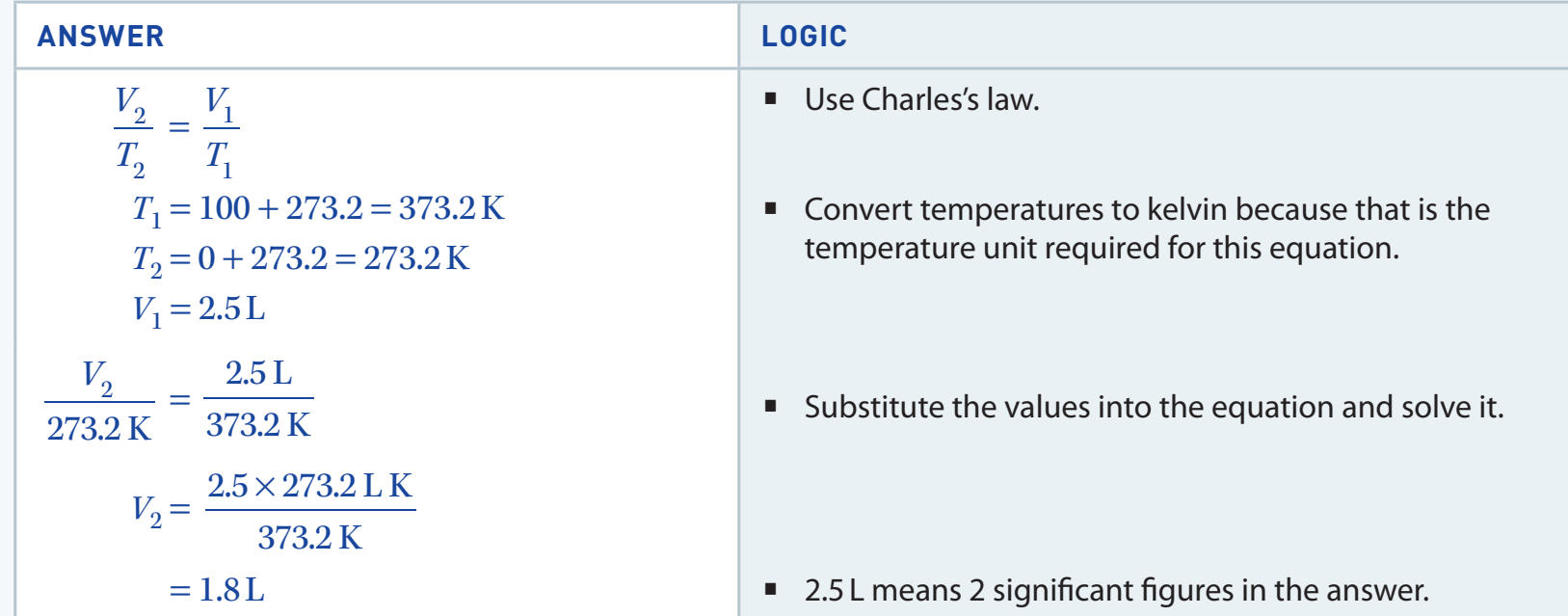
- Caption: Conversion from Celsius to Kelvin.
Units and Measurements for Gas Properties
Introduction to Units
Standard units: Fundamental for achieving accuracy and consistency in scientific communication.
Pressure Units
-
Atmosphere (atm)
-
Pascal (Pa) and Kilopascal (kPa): 1 kPa is equal to 1000 Pa.
-
Millimetres of Mercury (mmHg)
-
Convert 1 atm = 101.325 kPa = 760 mmHg.
Temperature Units
- Kelvin (K)
- Conversion Formula: Kelvin = Celsius + 273.15
Volume Units
- Litre (L) and Millilitre (mL)
Amount of Gas
- Mole: Associated with Avogadro's number.
Unit Conversions
- Celsius to Kelvin Conversion: Add 273.15.
- Example: Convert 25°C to Kelvin:
Measurement Contexts
- Pressure Measuring Devices: Barometers and manometers.
- Temperature Measuring Devices: Thermometers and thermocouples.
Conceptual Table
| Unit Type | Units | Context of Use |
|---|---|---|
| Pressure | atm, kPa | General, precise measurements |
| Temperature | K | Thermodynamic calculations |
Takeaways:
- Mastering conversion formulae is essential for exams.
- Each unit ensures the consistency necessary for scientific communication.
Kinetic Molecular Theory of Gases
Introduction to Kinetic Molecular Theory
- The Kinetic Molecular Theory (KMT) enhances our understanding of gas behaviour by describing particle motion.
Core Assumptions
-
Constant, Random Motion: Particles move continuously and unpredictably.
-
Elastic Collisions: Total energy in the system remains constant.
-
Negligible Volume: The volume of particles is insignificant compared to the space they occupy.
-
No Intermolecular Forces
-
Relationship Between Kinetic Energy and Temperature
Relation to Gas Laws
| Gas Law | KMT Assumption & Example |
|---|---|
| Boyle's Law | Pressure influenced by collisions, observed in a syringe. |
| Charles's Law | Volume-temperature increase in hot air balloons. |
Remember: Always use Kelvin for accurate temperature calculations.
Exam Tips
- Consistently use Kelvin.
- Ensure unit consistency (litres, kPa).
- Memorise primary formulae for effective understanding.
500K+ Students Use These Powerful Tools to Master Gas Properties and Laws For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
136 flashcards
Flashcards on Gas Properties and Laws
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Gas Properties and Laws
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes10 questions
Exam questions on Gas Properties and Laws
Boost your confidence with real exam questions.
Try Chemistry Questions3 exams created
Exam Builder on Gas Properties and Laws
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Gas Properties and Laws
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Gas Properties and Laws you should explore
Discover More Revision Notes Related to Gas Properties and Laws to Deepen Your Understanding and Improve Your Mastery
