Photo AI
Last Updated Sep 24, 2025
Alkanes Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Alkanes quickly and effectively.
432+ students studying
Alkanes
Alkanes are saturated hydrocarbons that exhibit single covalent bonds between carbon atoms. This structural characteristic imparts high stability, making alkanes a fundamental topic in organic chemistry.
Key Definitions
-
Alkane: Saturated hydrocarbons distinguished by the presence of single covalent bonds.
infoNote- Alkane: Hydrocarbons characterised solely by single carbon-carbon bonds.
-
Saturated Hydrocarbons:
- Hydrocarbons composed exclusively of single bonds, maximising hydrogen atom content per carbon atom.
Introduction to Alkanes
- Alkanes follow the general formula .
- They play a vital role in fuel production, offering significant energy release upon combustion.
- Alkanes are essential building blocks in organic chemistry, with properties varying based on chain length and branching.
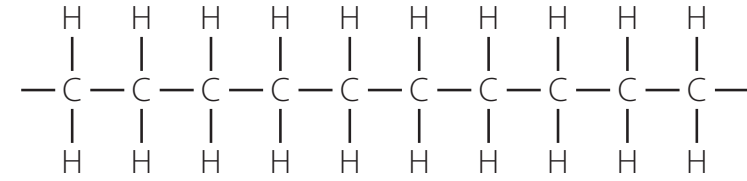
Physical Characteristics
- Alkanes are colourless and odourless.
- Small alkanes (e.g., methane, ethane) are gases, medium-sized alkanes (e.g., hexane, heptane) are liquids, while larger alkanes (e.g., decane) are solids.
Role in Fuel Production
- Common reaction:
- Fuels such as petrol and diesel are derived from alkanes.
Alkanes are crucial to the energy industry due to their high heat output.

Industrial Importance
- Alkanes are modified to produce compounds like ethylene.
- Catalytic techniques enhance combustion productivity, although they release carbon dioxide, leading to efforts to reduce environmental impact.
Comprehending the role of alkanes is vital for organic chemistry principles and industrial applications.
Bonding in Alkanes
- Alkanes contain only single bonds, rendering them saturated and stable.
- Sigma bonds (σ) are formed through sp³ hybridisation, contributing to the structure's stability and resulting in a tetrahedral geometry.
- Intramolecular and Intermolecular Forces:
- Intramolecular bonds maintain molecular structure.
- Weak intermolecular van der Waals forces influence physical properties such as boiling points.
Physical Properties
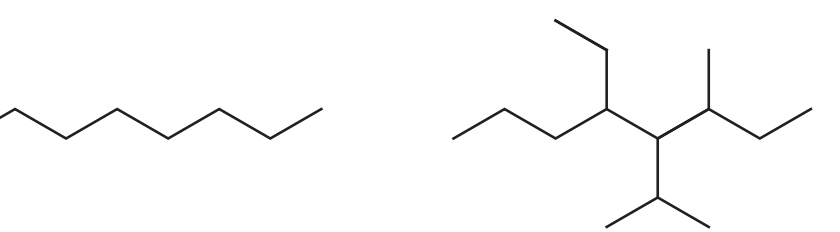
- Molecular size affects boiling/melting points; larger molecules possess higher points due to increased van der Waals forces.
- Branching decreases boiling/melting points.
Volatility and Density
- Volatility decreases as molecular mass increases.
- Density generally rises with chain length due to more compact molecular packing.

Functional Groups in Alkanes
- Alkanes Lack Functional Groups:
- Their simple structures contribute to stability.
- They primarily undergo combustion and substitution reactions.
Stability ensures predictable reactions but limits chemical versatility.
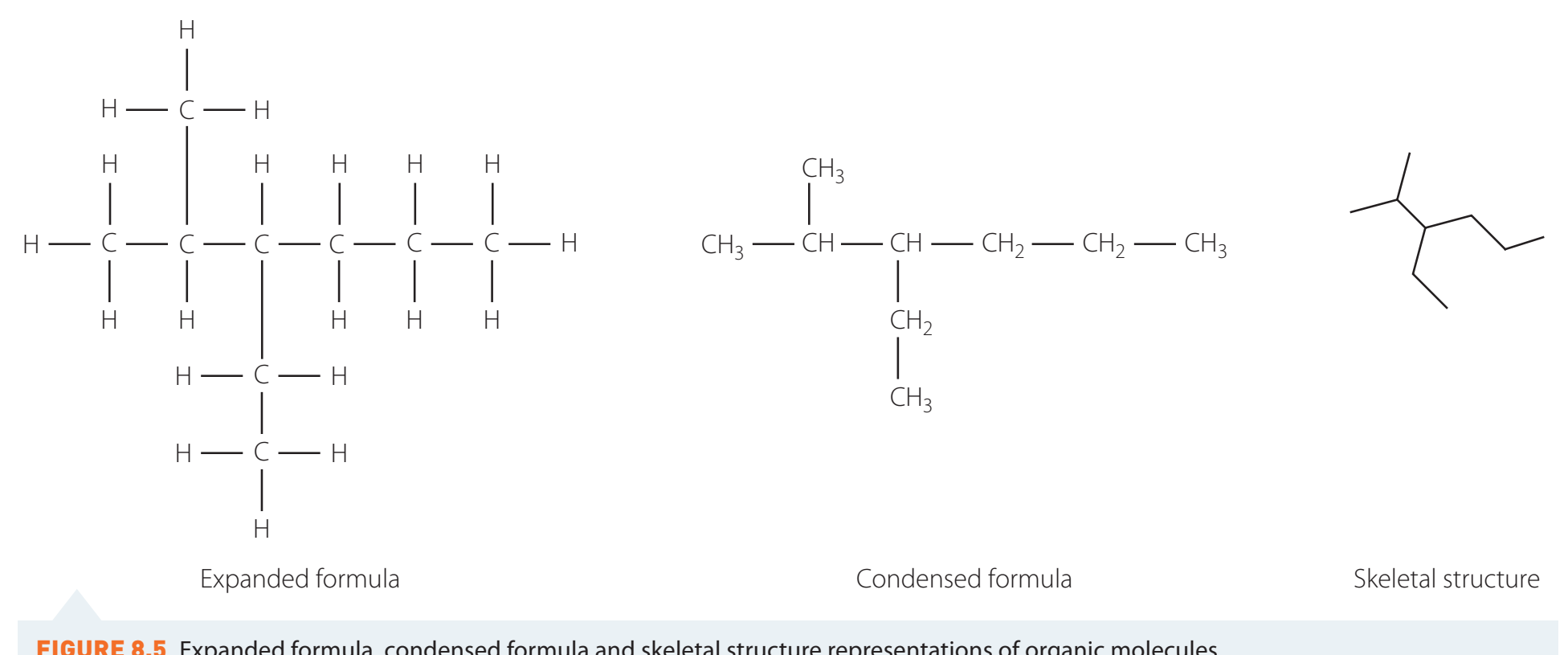
Formulae
- Structural Formula:
- Demonstrates atom arrangement.
- Molecular Formula:
- Lists atom quantities.
- Formulating involves determining the root name and employing to represent hydrogen content.

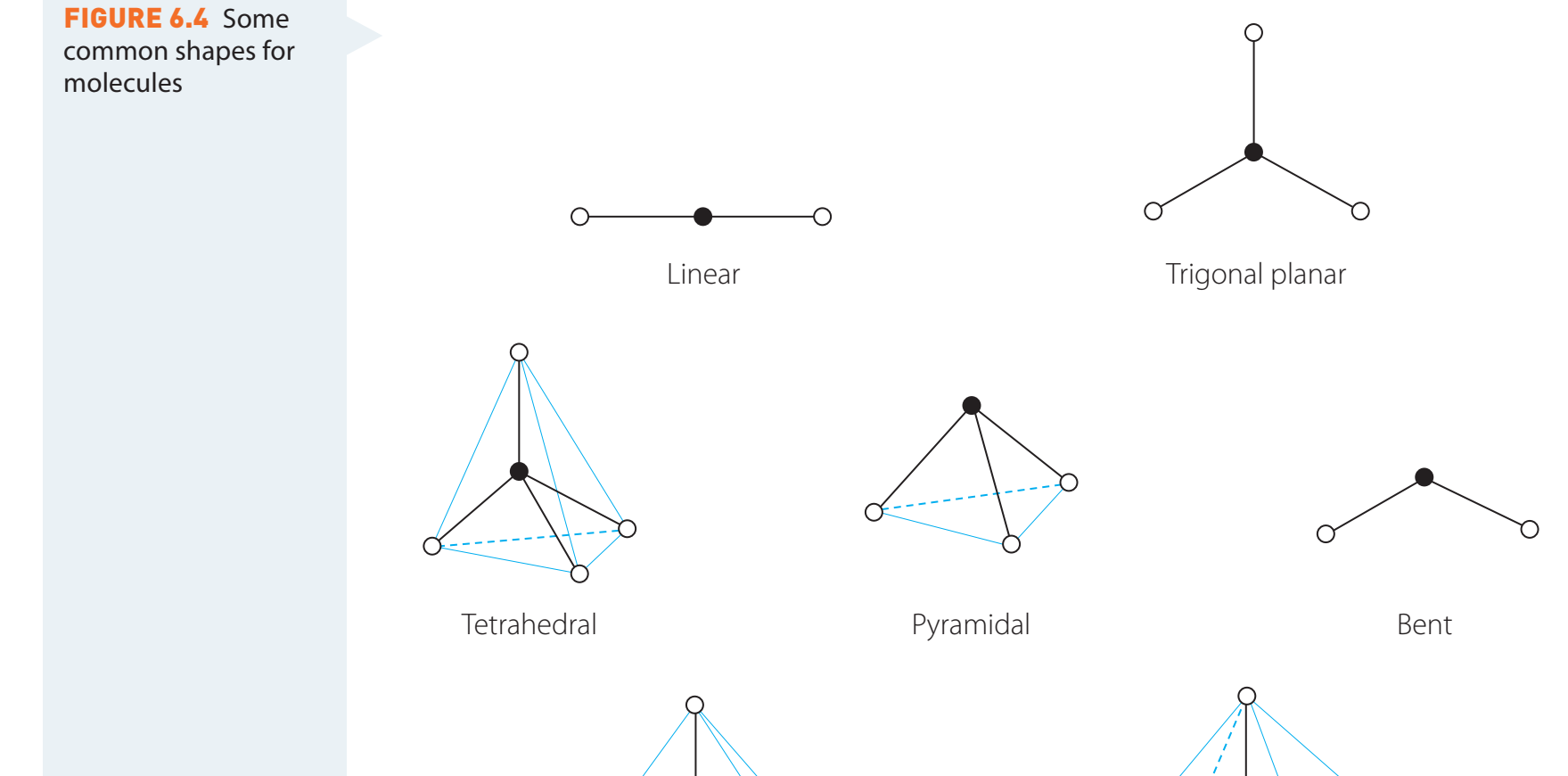
Model Construction
- Models visually depict three-dimensional atomic interactions.
- Ball-and-stick models, though potentially misrepresenting bond angles, facilitate comprehension.
Practice Problems and Solutions:
Problem 1: Identify and Draw
Task: Illustrate pentane's structural and molecular formulas.
Solution:
- Molecular formula: C₅H₁₂
- Structural formula: CH₃-CH₂-CH₂-CH₂-CH₃
- Ensure all carbon atoms have four bonds each to satisfy valency
Problem 2: Boiling Points
Task: Compare the boiling points of butane and 2-methylpropane.
Solution:
- Butane (linear structure) has a higher boiling point than 2-methylpropane (branched structure)
- This is because the linear structure allows for stronger van der Waals forces between molecules, requiring more energy to separate
Problem 3: Combustion Reaction
Task: Formulate the balanced combustion equation for hexane.
Solution:
- Unbalanced: C₆H₁₄ + O₂ → CO₂ + H₂O
- Balanced: C₆H₁₄ + 9½O₂ → 6CO₂ + 7H₂O
- Alternatively: 2C₆H₁₄ + 19O₂ → 12CO₂ + 14H₂O (with whole numbers)

Problem 4: Combustion Types in Octane
Task: Differentiate between complete and incomplete combustion.
Solution:
- Complete combustion: C₈H₁₈ + 12½O₂ → 8CO₂ + 9H₂O
- Occurs with excess oxygen
- Produces carbon dioxide and water only
- Incomplete combustion: C₈H₁₈ + limited O₂ → mixture of CO₂, CO, C (soot), H₂O
- Occurs with insufficient oxygen
- Produces carbon monoxide, carbon (soot), and/or unburnt fuel in addition to CO₂ and H₂O

500K+ Students Use These Powerful Tools to Master Alkanes For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
293 flashcards
Flashcards on Alkanes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards37 questions
Exam questions on Alkanes
Boost your confidence with real exam questions.
Try Chemistry Questions4 exams created
Exam Builder on Alkanes
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Alkanes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alkanes you should explore
Discover More Revision Notes Related to Alkanes to Deepen Your Understanding and Improve Your Mastery
