Photo AI
Last Updated Sep 24, 2025
Carbon Bonding Fundamentals Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Carbon Bonding Fundamentals quickly and effectively.
460+ students studying
Carbon Bonding Fundamentals
Carbon is fundamental to organic chemistry due to its exceptional capacity to form stable covalent bonds with a wide range of elements, including itself. This ability leads to the creation of complex and versatile compounds essential for both life and industrial applications.
Tetravalence of Carbon
- Tetravalence: Refers to carbon's potential to form four covalent bonds, allowing the construction of a diverse range of structures.
- Enables the development of various compounds and materials, e.g., elongated chain plastics and aromatic rings like benzene.
Tetravalence: Carbon's ability to form four bonds enables it to produce various essential industrial materials.
Hybridisation
Definition
- Hybridisation: The merging of orbitals results in unique molecular geometries.
- Example: Orbital mixing allows carbon to assume various shapes, similar to arranging LEGO pieces into different configurations.
Hybridisation: The blending of orbitals determines the form and function of molecules, akin to using a LEGO kit to create diverse structures.
Types of Hybridisation
sp³ Hybridisation
- Tetrahedral Shape: Consists of four equivalent sp³ hybrid orbitals with bond angles of .
- Example: Methane (), which features a three-dimensional, tetrahedral configuration.
sp³ Hybridisation: Generates a tetrahedral shape with four single bonds, serving as a foundational element for numerous substances.
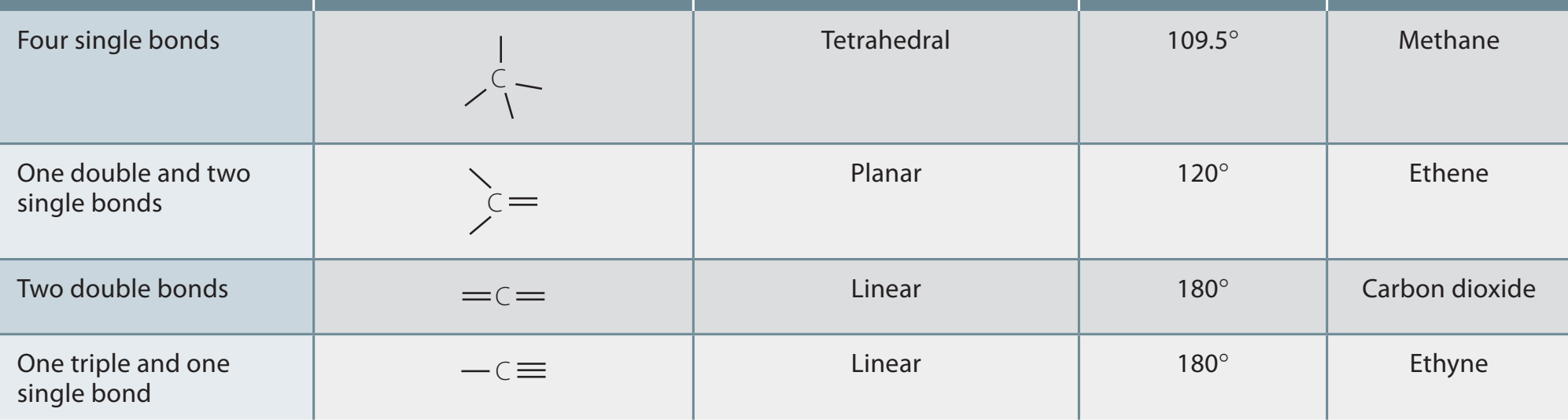
sp² Hybridisation
- Trigonal Planar Shape: Involves three equivalent sp² orbitals and one unhybridised p orbital, resulting in bond angles around .
- Example: Ethene ().
sp² Hybridisation: Forms a trigonal planar shape from three single bonds and one double bond, important in creating unsaturated compounds.
sp Hybridisation
- Linear Shape: Consists of two sp hybrid orbitals created by combining one s orbital with one p orbital, resulting in bond angles of .
- Example: Ethyne ().
sp Hybridisation: Produces a linear structure from one single and one triple bond, essential for aligning carbon atoms in a straight line.
Importance of Bond Angles
- The molecular shape significantly affects chemical reactivity and interactions.
- Bond angles, dictated by hybridisation, influence molecular orientation and reactivity.
- Example: In pharmaceuticals, the specific shape and angle of molecular bonds are crucial for drug efficacy.
Covalent Bonding and Intermolecular Forces
Covalent Bonds
- Covalent Bonds: Involve the sharing of electron pairs between atoms, forming stable molecular structures akin to alkanes.
Covalent Bonds: Electron pair sharing creates stable molecular structures, similar to how magnets exhibit temporary attraction.
Van der Waals Forces
- Alkanes exhibit weak dispersion forces, essential for determining physical properties like boiling points.
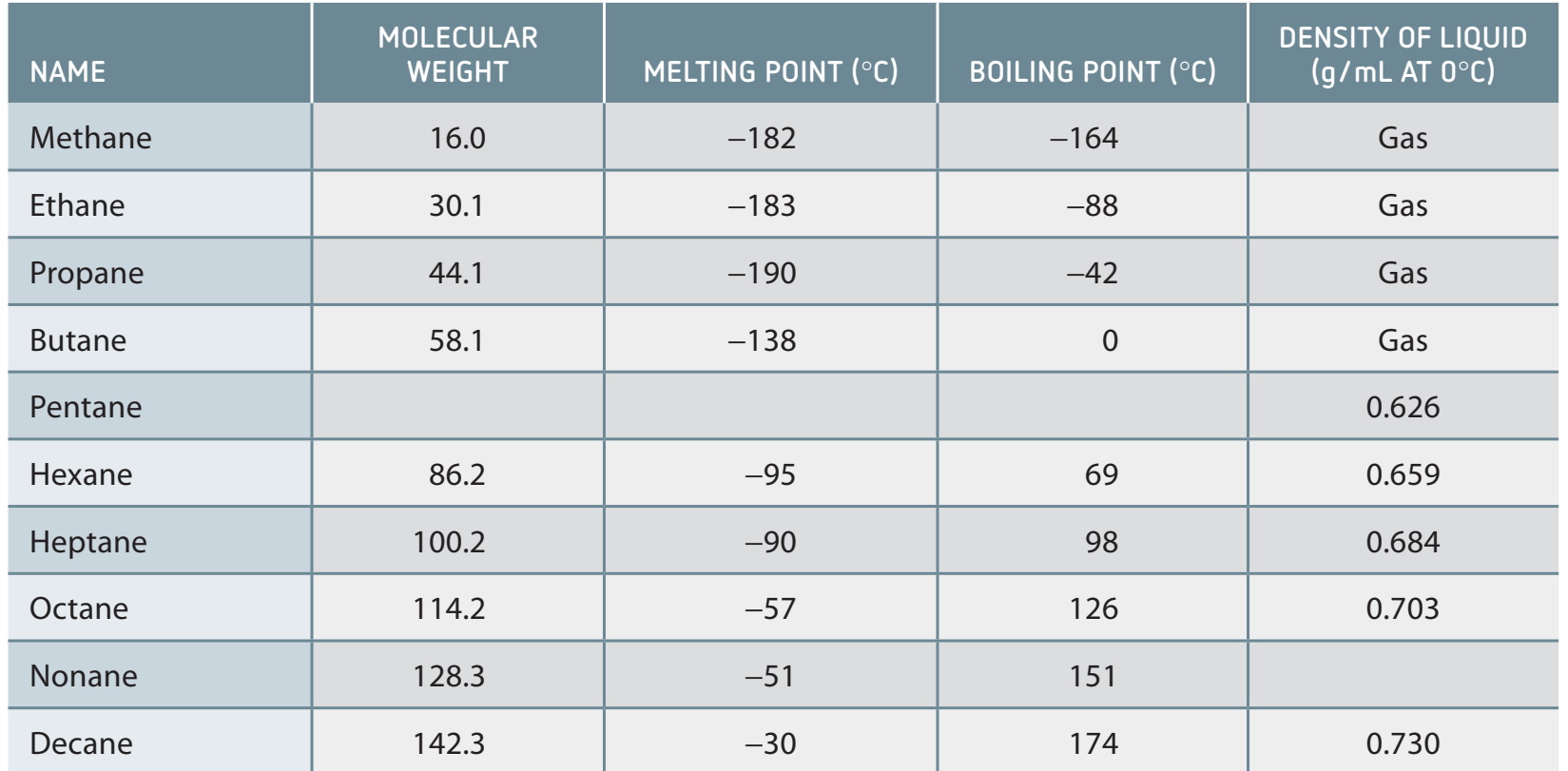
- These forces increase with chain length, affecting boiling and melting points.
- Molecular branching modifies boiling points by changing surface area contact.
Alkanes: A Homologous Series
- Homologous Series: A group of compounds with a similar general formula, differing by CH units.
- Examples: Methane (CH), Ethane (CH), Propane (CH).
Functional Groups in Alkenes and Alkynes
Effect on Reactivity
- Functional groups can modify chemical reactivity and physical properties.
- Example: Hydroxyl Group (-OH) can engage in hydrogen bonds, enhancing reactivity.
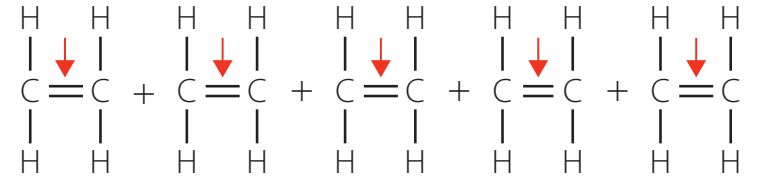
Geometric Isomerism
- Restricted rotation around double bonds leads to cis-trans isomers, which are significant for both biological and material properties.

Worked Examples
Example 1: Determining Bond Angles in Propane Propane has a molecular formula of C₃H₈. Each carbon atom in propane exhibits sp³ hybridisation, resulting in tetrahedral geometry with bond angles of approximately 109.5°.
Example 2: Comparing Molecular Rotation In ethene (C₂H₄), the sp² hybridised carbon atoms form a rigid double bond that prevents rotation. This restriction creates distinct cis and trans isomers. In contrast, ethyne (C₂H₂) with sp hybridisation has a linear structure with no possibility for cis-trans isomerism.
Example 3: Predicting Boiling Points When comparing straight-chain and branched alkanes:
- n-butane (straight chain, C₄H₁₀): boiling point = -0.5°C
- iso-butane (branched, C₄H₁₀): boiling point = -11.7°C
The straight-chain molecule has a higher boiling point because it has greater surface area for van der Waals interactions.
Practice Questions with Solutions
-
Question: Determine the bond angles for propane using knowledge of sp³ hybridisation.
Solution: In propane (C₃H₈), all carbon atoms are sp³ hybridised. This creates a tetrahedral arrangement around each carbon atom with bond angles of approximately 109.5°. The C-C-C bond angle may deviate slightly from this ideal value due to steric interactions between hydrogen atoms on adjacent carbon atoms.
-
Question: Compare the potential for molecular rotation in Ethene and Ethyne, and explain how this impacts their chemical properties.
Solution: In ethene (C₂H₄), the carbon atoms are sp² hybridised, forming a double bond that restricts rotation around the C=C axis. This restriction leads to geometric isomerism (cis-trans). In ethyne (C₂H₂), the carbon atoms are sp hybridised with a triple bond and linear geometry. The triple bond also prevents rotation, but due to its linear geometry, ethyne cannot exhibit geometric isomerism. These rotational restrictions affect reactivity patterns: ethene can undergo addition reactions at specific spatial orientations, while ethyne's linear structure allows for symmetrical addition from multiple directions.
500K+ Students Use These Powerful Tools to Master Carbon Bonding Fundamentals For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
293 flashcards
Flashcards on Carbon Bonding Fundamentals
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Carbon Bonding Fundamentals
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes37 questions
Exam questions on Carbon Bonding Fundamentals
Boost your confidence with real exam questions.
Try Chemistry Questions4 exams created
Exam Builder on Carbon Bonding Fundamentals
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Carbon Bonding Fundamentals
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Carbon Bonding Fundamentals you should explore
Discover More Revision Notes Related to Carbon Bonding Fundamentals to Deepen Your Understanding and Improve Your Mastery
