Photo AI
Last Updated Sep 24, 2025
Halogenated Organic Compounds Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Halogenated Organic Compounds quickly and effectively.
391+ students studying
Halogenated Organic Compounds
Definition and Structure
Halogenated Organic Compounds: These are hydrocarbons where one or more hydrogen atoms are substituted with halogen atoms, such as bromine, chlorine, fluorine, and iodine. This modification significantly alters properties like size, polarity, and reactivity. These compounds are vital in numerous industrial and synthetic applications but also present significant environmental and safety challenges.
Halogenated Organic Compounds: Hydrocarbons with hydrogen atoms replaced by halogen atoms.
Key Functional Groups and Halogen Position
-
Alkyl Halides (R-X):
- Primary relevance in organic chemistry.
- Halogen-carbon bonds affect molecular reactivity and stability.
-
Positioning of Halogens:
- Terminal: Located at chain ends, influencing end-group reactivity.
- Internal: Positioned within the chain, affecting stability and branching.
-
Notable Halogens: Fluorine, chlorine, bromine, and iodine each significantly modify a molecule's reactivity and polarity.
Comparison with Other Hydrocarbons
- Differences:
- Atomic sizes of halogens increase molecular dimensions.
- Polarity increases due to halogens' electron-withdrawing effects within C-X bonds.
- Altered reactivity compared to non-halogenated hydrocarbons.
The C-X bond enhances polarity in halogenated compounds.
Industrial and Synthetic Significance
-
Applications:
- Solvents: Essential in cleaning and industrial contexts.
- Refrigerants: Critical in systems requiring specific thermodynamic properties.
- Pharmaceuticals: Form integral components in drug design.
-
Synthetic Importance: Crucial in organic synthesis, enabling construction of complex molecular structures.
Halogenated compounds are indispensable in many industries due to their unique chemical properties.
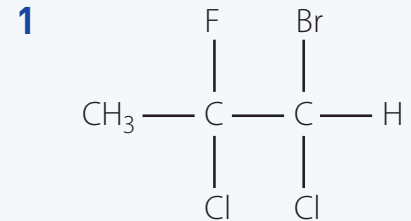
Introduction to IUPAC Nomenclature
IUPAC Nomenclature: Crucial for consistency and clarity in naming chemical compounds, ensuring precise communication, particularly for carbon chains up to C8.
Nomenclature Rules
Identifying the Longest Carbon Chain
- Determine the longest chain: Include functional groups such as halogens.
- Example: For 2-Chloropropane, select the three-carbon chain, accounting for chlorine.
Numbering the Carbon Chain
- Begin numbering nearest to a substituent (halogen) to achieve the lowest locants.
- Correct numbering is crucial to maintain compound identity.
Incorrect locants can lead to entirely different substances.
- Comparison Table:
- Correct: 2-Chloropropane
- Incorrect: 3-Chloropropane
Ordering Halogen Prefixes
- Arrange prefixes alphabetically (e.g., 1-Bromo, 2-Chloro) for systematic recognition.
Common Errors and Misunderstandings
Errors in locants and prefix issues: Always utilise di-, tri- for multiple identical halogens.
Overview of Nucleophilic Substitution Reactions
Nucleophilic Substitution Reactions: A mechanism where a nucleophile substitutes a leaving group, converting halogenated compounds.
- SN1 vs. SN2 Reactions:
- SN1 (Unimolecular):
- Two-step process involving a carbocation intermediate, favoured by tertiary substrates.
- SN2 (Bimolecular):
- Single-step direct substitution, best with primary substrates.
- SN1 (Unimolecular):

For SN1, tertiary substrates facilitate carbocation stability.
Mechanistic Details
SN1 Mechanism
- Steps:
- Carbocation Formation: The leaving group exits, creating a carbocation.
- Nucleophilic Attack: Nucleophile attacks to form the product.
SN2 Mechanism
- Steps:
- Direct Attack: Nucleophile attacks carbon bound to the leaving group.
- Transition State: Formation of a high-energy state.
Primary substrates are more favourable for SN2 reactions.

Introduction to Alcohol Production via Hydrolysis
Hydrolysis: A chemical reaction where a halogen atom is replaced by a hydroxyl (-OH) group, producing alcohols.
-
General Reaction Formula:
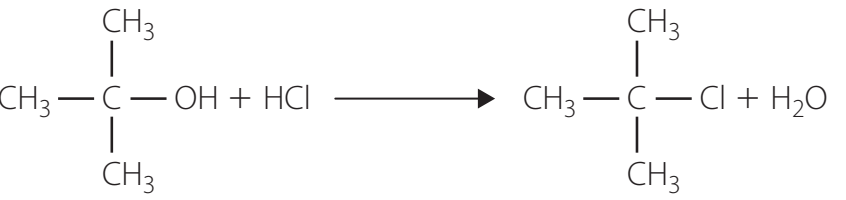
Hydrolysis: Substituting halogens with hydroxyl groups to create alcohols.
Mechanism Analysis
Stepwise Reaction Mechanism
- Nucleophilic Attack: Initiated by a hydroxide ion.
- Transition State: Temporary high-energy configuration.
- Leaving Group: Halogen departs, stabilised by electron gain.

Factors Affecting Hydrolysis
- Solvent Effects: Polar protic solvents boost nucleophilic attacks.
- Catalyst Role: Acids/bases reduce activation energy.
- Temperature Effect: Elevates reaction rate.
Conditions like solvent choice and catalysts significantly influence hydrolysis processes.
Environmental Impact of Halogenated Compounds
Ozone Depletion
- Chlorine-containing halogenated compounds: Major contributors to ozone layer depletion.
- CFCs (Chlorofluorocarbons): Liberate chlorine radicals, degrading ozone.
- Bromine compounds: More potent than chlorine in causing depletion.
Halogenated radicals have a severe impact on the ozone layer.
Safety Measures for Handling and Disposal
Handling Precautions
- Protective Gear: Gloves, goggles, and lab coats are recommended.
- Ventilation: Crucial to avoid inhalation of fumes.
Using greener solvent alternatives can minimise risks.
Disposal Practices
- Safe Methods: Incineration in compliance with legal requirements.
- Avoidance: Never dispose of in drains to prevent environmental damage.
Regulatory Frameworks and Green Chemistry
Montreal Protocol
- Overview: Global agreement to phase out ozone-depleting substances.
- Effectiveness: Resulted in significant emission reductions aiding ozone recovery.
Green Chemistry Alternatives
- Research Focus: Ongoing studies for safer alternatives.
- Green Solvents: Increasingly adopted as sustainable options.

Exam Tips
- Mnemonic: "Alphabet before Number" helps facilitate systematic molecular naming.
- Engaging in interactive learning and hazard recognition and disposal enhances exam preparation.
Practice Problems with Solutions
Reaction Identification
-
Problem: Identify whether the reaction of 2-chloro-2-methylpropane with sodium hydroxide in aqueous solution proceeds via SN1 or SN2 mechanism.
Solution: This reaction proceeds via SN1 mechanism because:
- The substrate is tertiary (2-chloro-2-methylpropane)
- Tertiary substrates form stable carbocations
- Aqueous solution favours the ionisation step in SN1
-
Problem: Predict the major product when 1-bromobutane reacts with sodium ethoxide in ethanol.
Solution: The major product is butyl ethyl ether (CH3CH2CH2CH2OCH2CH3). This proceeds via SN2 mechanism because:
- 1-bromobutane is a primary alkyl halide
- Sodium ethoxide is a strong nucleophile
- The reaction occurs in one step with inversion of configuration
-
Problem: How would temperature affect the rate of hydrolysis of chlorobenzene?
Solution: Increasing temperature would slightly increase the rate, but chlorobenzene is resistant to hydrolysis due to:
- The C-Cl bond being stabilised by resonance with the benzene ring
- Higher activation energy required
- A catalyst would be necessary for significant reaction
Interactive Quiz with Answers
- What measures should be taken in handling halogenated compounds?
- A) Wear gloves
- B) Wear gloves, goggles, ensure ventilation
- C) Work without protection
Answer: B) Wear gloves, goggles, ensure ventilation
- Impact of CFCs on ozone?
- A) Repair ozone
- B) Release radicals degrading ozone
- C) Have no effect
Answer: B) Release radicals degrading ozone
- Bioaccumulation effect on biodiversity?
- A) Strengthens it
- B) Alters food chains, impacting biodiversity
- C) Unrelated to biodiversity
Answer: B) Alters food chains, impacting biodiversity
- Goal of the Montreal Protocol?
- A) Increase CFC use
- B) Phase out ozone-depleting substances
- C) Ban all chemicals
Answer: B) Phase out ozone-depleting substances
500K+ Students Use These Powerful Tools to Master Halogenated Organic Compounds For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
293 flashcards
Flashcards on Halogenated Organic Compounds
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Halogenated Organic Compounds
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes37 questions
Exam questions on Halogenated Organic Compounds
Boost your confidence with real exam questions.
Try Chemistry Questions4 exams created
Exam Builder on Halogenated Organic Compounds
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Halogenated Organic Compounds
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Halogenated Organic Compounds you should explore
Discover More Revision Notes Related to Halogenated Organic Compounds to Deepen Your Understanding and Improve Your Mastery
