Photo AI
Last Updated Sep 24, 2025
Benzene: Fundamental Aromatic Hydrocarbon Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Benzene: Fundamental Aromatic Hydrocarbon quickly and effectively.
325+ students studying
Benzene: Fundamental Aromatic Hydrocarbon
Overview of Benzene
- Benzene: A basic aromatic hydrocarbon with the molecular formula . Its planar, hexagonal structure is essential for understanding aromatic stability.
- Historical Context:
- In 1865, Friedrich August Kekulé introduced the ring structure of benzene, marking a significant advancement in understanding chemical stability and resonance.
- This discovery paved the way for progress in chemical synthesis and industrial applications, including pharmaceuticals and polymers.
Key Terms and Definitions
-
Aromaticity:
- Aromaticity: The stability of benzene due to its cyclic, planar structure with delocalised electrons. This results in lower reactivity in specific reactions, such as resistance to hydrogenation.
-
Resonance:
- Involves multiple structures that exhibit electron delocalisation. This maintains benzene's stability and uniform bond lengths, as demonstrated by spectroscopy.
infoNoteResonance: Describes benzene as various forms that depict delocalised electrons and consistent bond lengths.
-
Kekulé Structures:
- Illustrate alternating single and double bond representations in benzene, which have evolved into modern models that enhance the understanding of its aromatic stability.
Important Diagrams
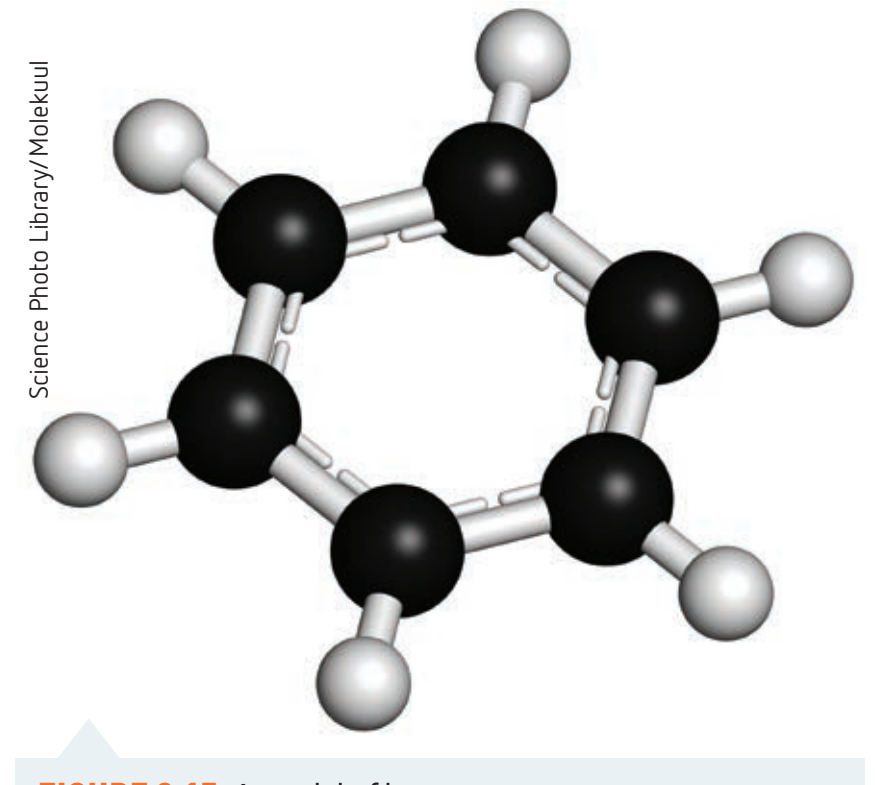 Depiction of benzene's hexagonal ring structure according to Kekulé's representation.
Depiction of benzene's hexagonal ring structure according to Kekulé's representation.
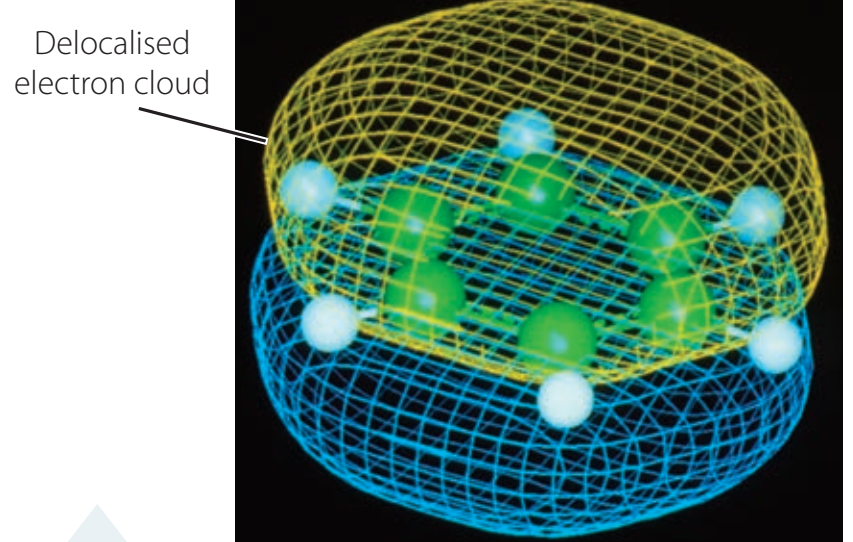 Visualisation of electron delocalisation in benzene emphasising resonance.
Visualisation of electron delocalisation in benzene emphasising resonance.
Benzene's Industrial Significance
-
Pharmaceuticals:
- Benzene derivatives are vital for medications such as aspirin and ibuprofen.
- Essential components in synthetic organic chemistry.
-
Polymers:
- Critical for materials like polystyrene, utilised in packaging and insulation.
Safety and Environmental Considerations
Toxicity Concerns: Benzene is highly toxic, requiring strict adherence to safety regulations.
- Environmental Impact:
- Regulations, such as EU directives, limit benzene emissions and encourage sustainable practices. Innovations aim to develop safer solvent alternatives.
Benzene in Industry
Overview
-
Polymers and Plastics:
- Polystyrene: Used in products like plastic cutlery and containers.
- Nylon: Found in fabrics and ropes; benzene plays a crucial role in its production.
-
Pharmaceuticals:
- Aspirin: A medication derived from benzene, highlighting its importance in healthcare.
-
Other Chemicals:
- Aniline and Phenol: Utilised in dye production and resin creation.
infoNotePhenol: A benzene derivative used in creating resins for various products.
Aromatic Stability and Resonance
-
Aromatic Stability:
- Achieved through a unique arrangement of electrons in resonance structures, resulting in lower energy states.
- Hückel's Rule: Benzene adheres to the 4n+2 π electron rule, possessing six π electrons.
infoNoteHückel's Rule Summary: Aromatic compounds must have 4n+2 π electrons. Benzene's six complies perfectly.
-
Resonance Energy:
- Denotes enhanced stability via electron delocalisation. Formula:
infoNotePractice Problem: Calculate the resonance energy of benzene:
If the hypothetical enthalpy for a structure with alternating single and double bonds is 150 kJ/mol and the experimental enthalpy is 84 kJ/mol, the resonance energy would be:
This positive value indicates benzene's enhanced stability due to resonance.
Electrophilic Aromatic Substitution (EAS)
-
EAS:
- Preserves benzene's aromatic stability, differing from typical alkene addition reactions.
-
Mechanism:
- Formation of a strong electrophile with catalysts like H₂SO₄ and AlCl₃.
- Creation of a σ-complex, where pi electrons stabilise benzene's structure.
infoNoteDeprotonation is essential to restore the aromatic ring; understanding this is crucial.
-
Common Reactions:
- Includes Nitration, Halogenation, Sulfonation, and Friedel-Crafts Reactions.
Naming Substituted Benzene Compounds
-
Purpose:
- Clarify systematic (IUPAC) and common naming conventions.

-
Mono- and Di-Substituted:
- Utilise ortho-, meta-, and para- designations for positions.
chatImportantActivators enhance reactivity while deactivators reduce it, impacting naming and reactivity.
-
Naming Exercise:
- Name the following compound: benzene with chlorine at positions 1 and 3.
- Solution: 1,3-dichlorobenzene or meta-dichlorobenzene
Exam Tips
- Grasp the concept of aromatic stability and resonance.
- Familiarise yourself with safety protocols for handling benzene.
- Practice naming and substitution patterns.
- Review mechanism diagrams to reinforce understanding.
By understanding benzene's significance and characteristics, students can better appreciate its role both in industry and chemical theory.
500K+ Students Use These Powerful Tools to Master Benzene: Fundamental Aromatic Hydrocarbon For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
293 flashcards
Flashcards on Benzene: Fundamental Aromatic Hydrocarbon
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards22 quizzes
Quizzes on Benzene: Fundamental Aromatic Hydrocarbon
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes37 questions
Exam questions on Benzene: Fundamental Aromatic Hydrocarbon
Boost your confidence with real exam questions.
Try Chemistry Questions4 exams created
Exam Builder on Benzene: Fundamental Aromatic Hydrocarbon
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Benzene: Fundamental Aromatic Hydrocarbon
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Benzene: Fundamental Aromatic Hydrocarbon you should explore
Discover More Revision Notes Related to Benzene: Fundamental Aromatic Hydrocarbon to Deepen Your Understanding and Improve Your Mastery
