Photo AI
Last Updated Sep 24, 2025
Addition Polymers Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Addition Polymers quickly and effectively.
238+ students studying
Addition Polymers
Have you ever wondered why plastic wrap stretches while water pipes remain rigid? This difference arises from addition polymers and their distinct properties.
Addition Polymers: Formed by the repetitive addition of monomer units.
Addition Polymerisation: Involves the use of unsaturated monomers such as alkenes, which join through the breaking of double bonds.
Addition Polymers: Created by the continuous addition of monomers. Addition Polymerisation: Employs unsaturated monomers like alkenes.
Understanding Addition Polymers
- Monomers containing double bonds connect to form polymer chains.
- The process is straightforward and efficient compared to alternative methods.
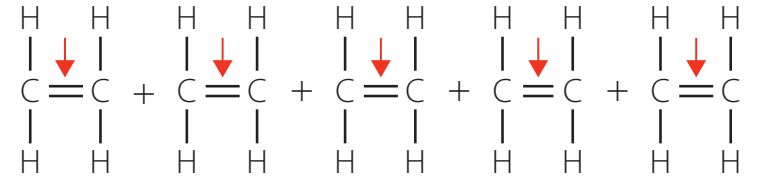
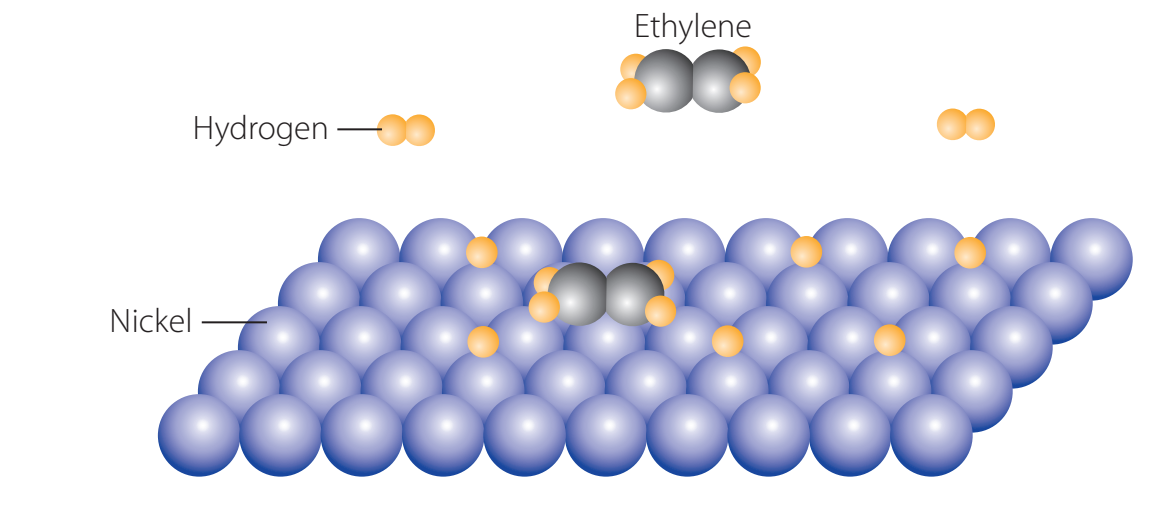
Role of Catalysts
- Catalysts enhance the rate and efficiency of polymerisation.
- Ziegler-Natta catalysts influence the polymer's molecular weight and branching.
Catalysts play a crucial role in accelerating the process and shaping polymer properties.
Structural Overview
| Polymer | Structure | Key Functional Groups |
|---|---|---|
| PE | None | |
| PVC | Chloride | |
| PS | Phenyl | |
| PTFE | C-F Bonds |
Functional groups are vital in determining properties such as chemical resistance and mechanical strength.
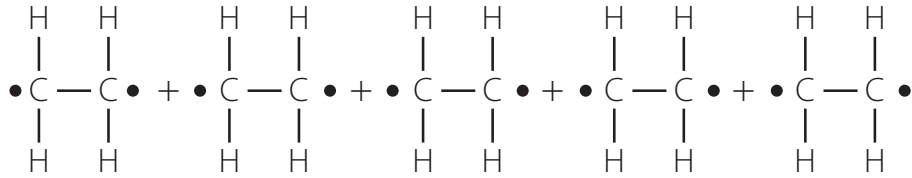
Polyethylene
Structure of Polyethylene
Polyethylene (PE): A widely used plastic formed by linking ethylene units in a chain.
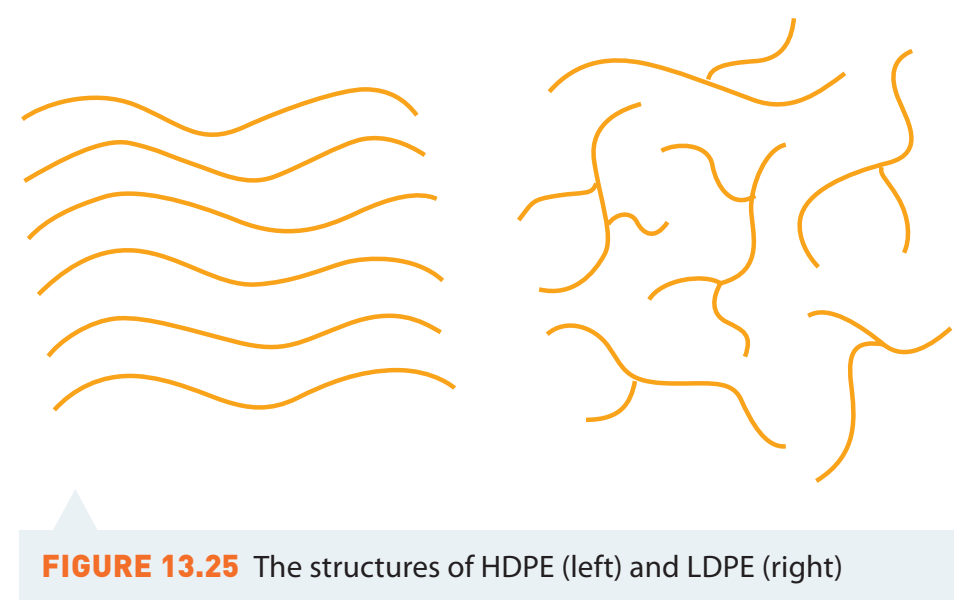
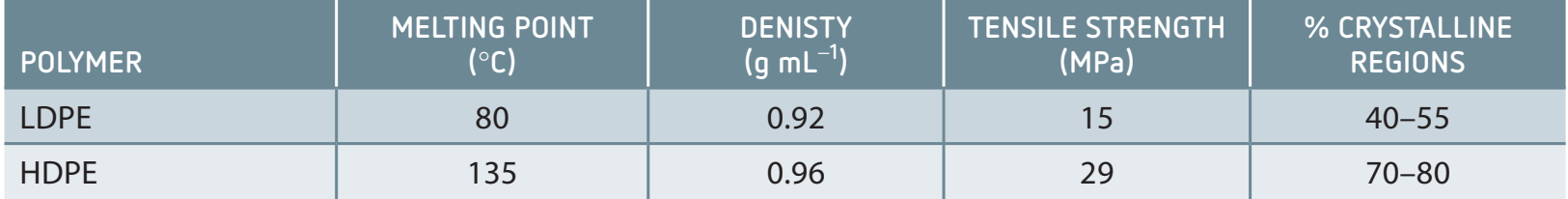
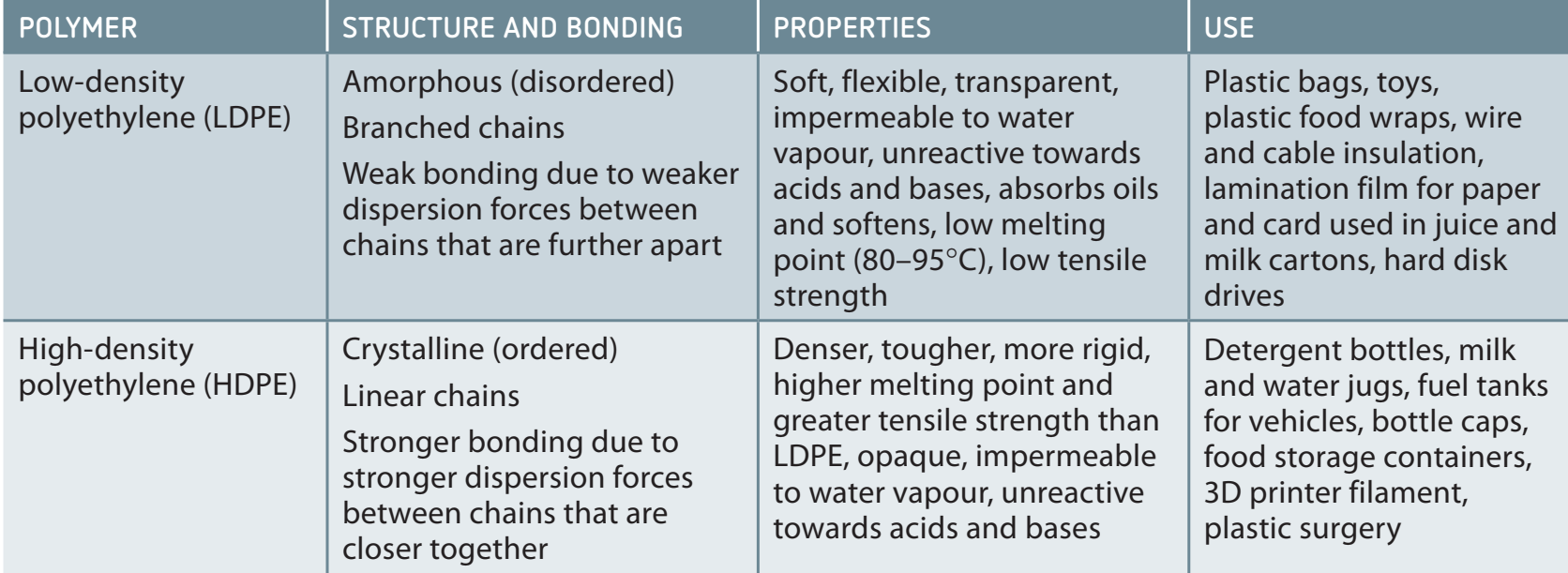
LDPE vs. HDPE
-
Low-Density Polyethylene (LDPE):
- Structure: Visualise LDPE as a tree with branches.
- Properties: Lower tensile strength, suitable for cling wraps and bags.
-
High-Density Polyethylene (HDPE):
- Structure: Imagine HDPE as a straight pole.
- Properties: High tensile strength, used in bottles and tanks.
Common Uses
- LDPE: Utilised for supermarket bags and cling wraps, commonly found at home.
- HDPE: Employed in milk jugs and detergent bottles.
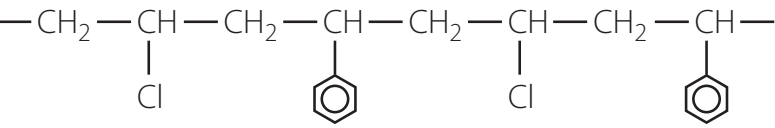
Polyvinyl Chloride (PVC)
Structure and Composition
Polyvinyl Chloride (PVC) is polymerised from vinyl chloride monomer. The chlorine atoms in PVC contribute to its resistance to fire and chemicals.
Forms of PVC
- Flexible PVC: Made pliable by plasticisers, used in garden hoses.
- Rigid PVC: Strengthened with stabilisers, suitable for pipes.
Properties and Uses
- Chemical Resistance: Ideal for industrial chemical containment.
- Durable: Common in construction, such as window frames.
Environmental Considerations
- Production releases harmful dioxins.
Effective management of PVC's environmental impact is essential.
Polystyrene (PS)
Structure of Polystyrene
Polystyrene: Derived from the monomer styrene. Its phenyl groups restrict chain mobility, providing rigidity.

Applications
- EPS (Expanded Polystyrene): Used in packaging and insulation.
- Solid PS (Crystal): Utilised for clear, rigid products.

Properties
- Brittleness: Tends to shatter under impact.
- Thermal Insulation: Air within EPS makes it an excellent insulator.
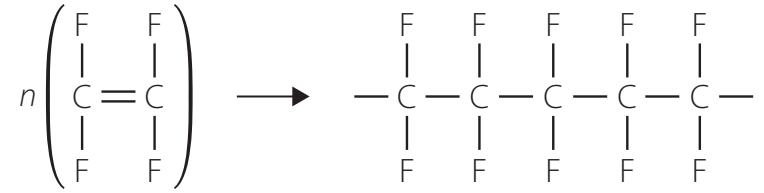
Polytetrafluoroethylene (PTFE)
Introduction to PTFE
PTFE, also known as Teflon, is renowned for its heat resistance. Discovered in 1938 by Roy Plunkett, it is a staple in non-stick cookware and industrial applications.
Properties
- Thermal Resistance: Crucial for high-temperature applications, such as cookware.
- Chemical Resistance & Non-Stick Surface: Attributed to strong carbon-fluorine bonds.

Applications of PTFE
- Cookware: Provides easy maintenance and cleaning.
- Electrical Insulators: Valued for dielectric properties.
Environmental Challenges and Recycling
Environmental Impact
- Polyethylene (PE): A major contributor to plastic pollution.
- Polyvinyl Chloride (PVC): Produces harmful emissions during manufacturing.
- Polystyrene (PS): Persistently remains in ecosystems, challenging to recycle.
- PTFE: Recycling complicated by its strong chemical bonds.
Sustainability and innovative recycling solutions are critical.
Recycling Advancements
- PE: Technological improvements enhance recovery processes.
- PVC: Innovations like Vinyloop improve recycling capabilities.
- PS: Chemical breakthroughs make component breakdown more efficient.
- PTFE: Pyrolysis offers promise for recovery.
Exam Tips
Identifying the properties of polymers aids in making informed material selections:
- For high thermal stability or chemical resistance, PTFE is the optimal choice.
- If flexibility is required, LDPE is the best candidate.
- For safe and effective pipe materials, consider PVC.
Key Takeaways for Exams:
- Comprehending properties is pivotal for application efficiency.
- Progress in recycling polymer materials is gaining traction.

500K+ Students Use These Powerful Tools to Master Addition Polymers For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
188 flashcards
Flashcards on Addition Polymers
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards16 quizzes
Quizzes on Addition Polymers
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Addition Polymers
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Addition Polymers
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Addition Polymers
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Addition Polymers you should explore
Discover More Revision Notes Related to Addition Polymers to Deepen Your Understanding and Improve Your Mastery
