Photo AI
Last Updated Sep 24, 2025
Polymer Properties and Structures Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Polymer Properties and Structures quickly and effectively.
465+ students studying
Polymer Properties and Structures
Introduction to Functional Groups
- Functional Groups: Specific atomic arrangements that determine the characteristics and reactions of molecules.
- Essential for the formation of polymers.
Understanding functional groups is crucial since they determine the behaviour and reactions of organic compounds.
Key Functional Groups in Polymer Formation
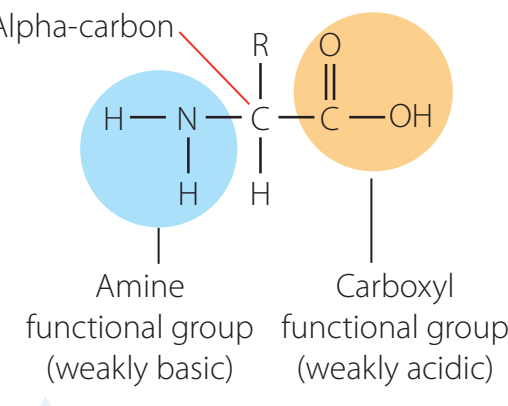
- Amino Group ():
- Composed of nitrogen bonded to hydrogen and carbon.
- Key in forming peptide bonds.
- Hydroxyl Group ():
- Consists of an oxygen atom bonded to a hydrogen atom.
- Important for hydrogen bonding; affects the elasticity of polymers.
- Carboxylic Acid Group ():
- Includes a carbon atom double-bonded to oxygen and an attached hydroxyl group.
- Critical in forming esters and amides.
- Acyl Chloride Group ():
- Consists of a carbonyl group bonded to chlorine.
- Utilised in polymer synthesis.
Polymerisation Processes
- Addition Polymerisation:
- Monomers connect without producing by-products.
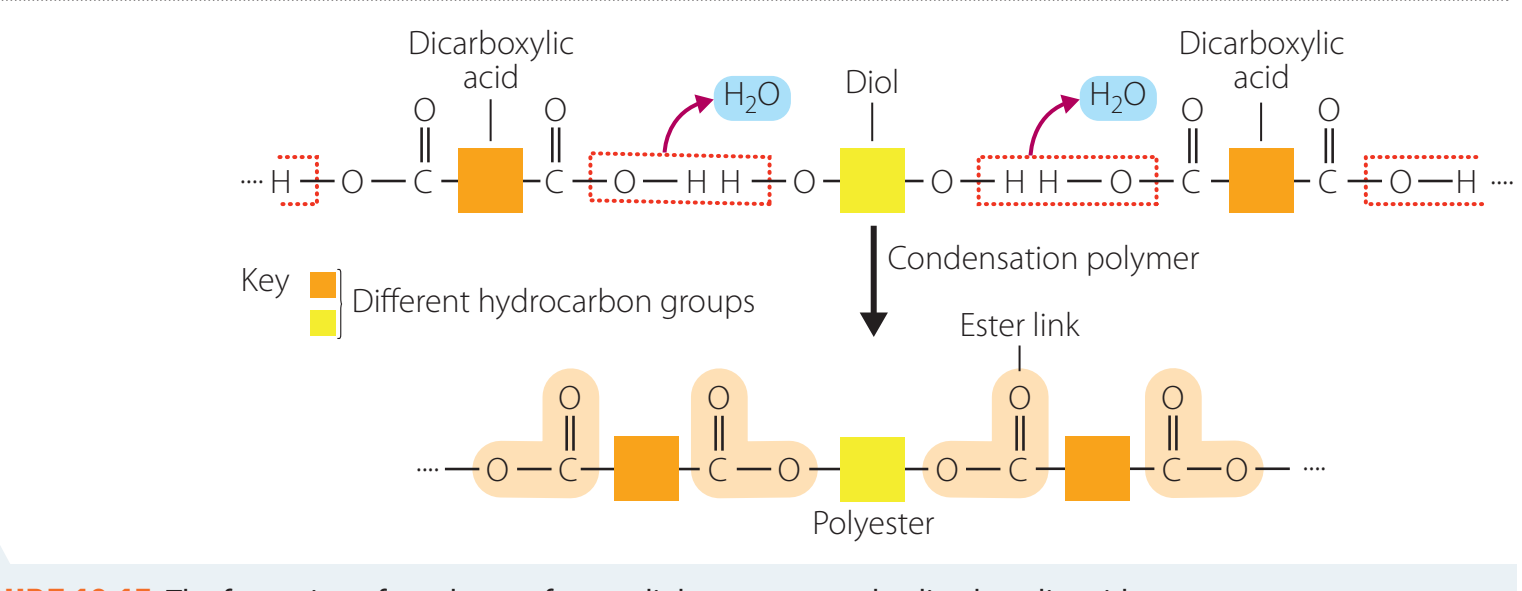
- Condensation Polymerisation:
- Monomers join, releasing small molecules such as water.
Role of Functional Groups:
- Influence polymerisation pathways (e.g., formation of polyamides involving acyl groups).
In condensation polymerisation, monomers bond and release a by-product such as water.
Addition polymerisation differs in that no by-product is generated.
Chemical Properties Influenced by Functional Groups
- Affect mechanical attributes like flexibility and strength.
- Hydroxyl Groups:
- Facilitate hydrogen bonding.
- Influence polymer resilience and flexibility.
Examples:
- Nylon: Known for strength and flexibility due to amide linkages.
- Proteins: Perform various functions owing to the presence of multiple functional groups.

Reactions Based on Functional Groups
-
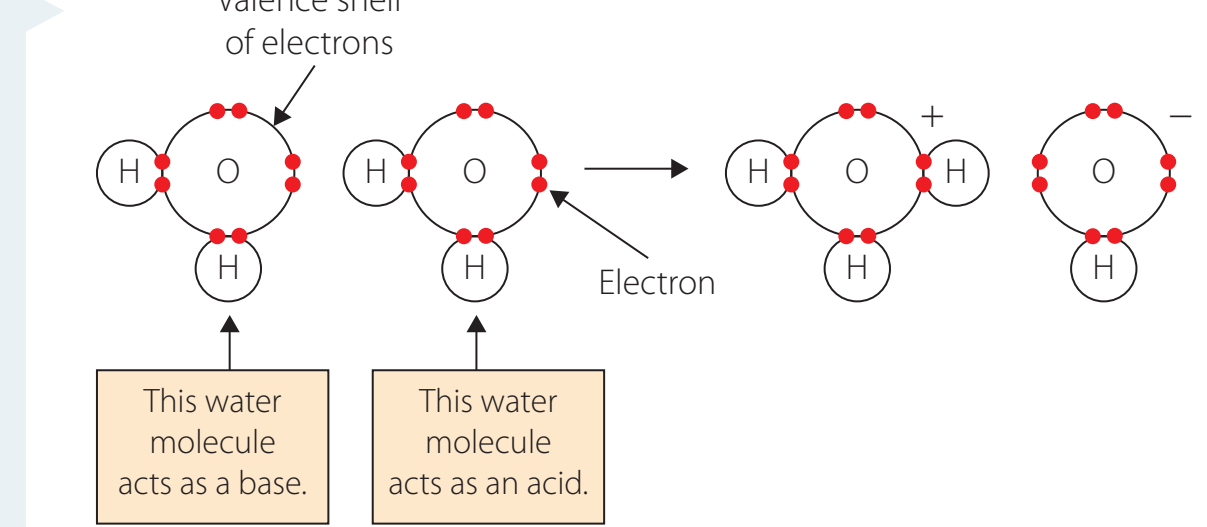
Acid-Base Reactions:
- Typically involve carboxylic and amino groups.
- Help maintain the stability of polymers.
-
Oxidation Reactions:
- May affect stability and strength.
Example:
- Oxidation of hydroxyl groups can influence polymer durability.
Properties of Organic Materials
Analysis of Properties Related to Molecular Structure
Strength and Biodegradability of Polymers
-
Crystallinity-Based Strength: The degree of crystallinity significantly influences a polymer's tensile strength.
- Examples:
- Polyethylene: Utilised in fuel tanks, noted for durability.
- Polystyrene: Used in foam packaging, requiring less rigidity.
infoNoteCrystallinity Defined: The alignment and structure within a polymer, enhancing molecular packing and increasing strength.
- Examples:
-
Impact of Branching:
- Linear Polymers: Greater density, superior strength.
- Branched Polymers: Lower density, enhanced flexibility.
Density and Structural Arrangement
- Effect of Polymer Chain Arrangement: Density is related to how closely chains pack together.
- Linear Chains: Promote compactness and increased density.
- Branched Chains: Result in greater flexibility.
Effects of Environmental Factors
- Temperature and Elasticity:
- Increasing temperature generally results in greater elasticity, while cooling increases rigidity.
Contrasting Chemical and Physical Properties
- Reactivity and Material Characteristics:
- PVC: Low reactivity; strong and flexible.
- LDPE: More reactive; selected for its elastic nature.
Industry Impact: Advances in biodegradable plastics have revolutionised industries by minimising environmental impact.
Overview of Synthetic Polymers
Synthetic polymers: Created artificially, often from petrochemicals.
Specific Applications
- Packaging: Lightweight and durable, suitable for bags and wraps.
- Technology: Used in insulation and flexible circuits for electronics.
- Automotive Parts: Incorporated in bumpers and dashboards due to their strength and impact resistance.
Types and Characteristics
- Polyethylene:
- Known for strength and flexibility, ideal for plastic bags and bottles.
- Polyvinyl Chloride (PVC):
- Offers chemical resistance, making it suitable for pipelines and cables.
- Teflon:
- Provides heat and chemical resistance, ideal for non-stick cookware.
Polymerisation Techniques
- Bulk Polymerisation: Produces high-purity polymers.
- Solution Polymerisation: Ensures good temperature control and uniformity.
- Emulsion Polymerisation: Enables the rapid creation of high molecular weight polymers.
Role of Additives
- Stabilisers: Enhance durability by preventing degradation.
- Plasticisers: Improve flexibility.
- Fillers: Increase toughness.
Structural Comparison
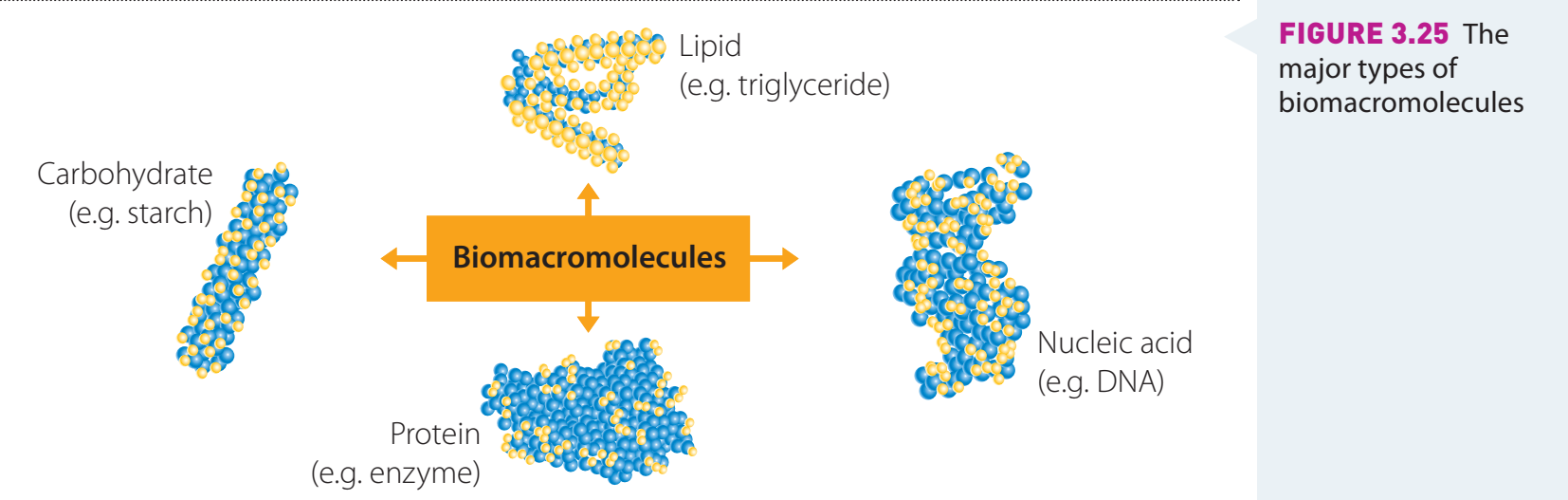
- Proteins: Chains of amino acids linked by peptide bonds.
- Carbohydrates: Composed of sugar blocks.
- Synthetic Polymers: Arranged like flexible lego bricks.
Property Analysis
Solubility and Reactivity
- Proteins: Solubility depends on polar amino acids presence.
- Carbohydrates: Solubility driven by hydroxyl groups.
- Synthetic Polymers: Resilience is determined by chemical bonds.
Strength and Elasticity
- Proteins: Cross-links provide strength.
- Carbohydrates: Cellulose imparts rigidity.
- Synthetic Polymers: Stretch due to the free movement of their chains.
Chain Interactions: Affect properties such as stretchiness and strength.
Environmental and Application Contexts
Biodegradability
- Proteins and Carbohydrates: Easily decompose.
- Synthetic Polymers: Some designed for rapid biodegradation.
Industrial Applications
- Proteins: Utilised in pharmaceuticals and dietary products.
- Carbohydrates: Integral to food production.
- Synthetic Polymers: Widely used in products like wraps and automotive components.
500K+ Students Use These Powerful Tools to Master Polymer Properties and Structures For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
188 flashcards
Flashcards on Polymer Properties and Structures
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards16 quizzes
Quizzes on Polymer Properties and Structures
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Polymer Properties and Structures
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Polymer Properties and Structures
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Polymer Properties and Structures
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Polymer Properties and Structures you should explore
Discover More Revision Notes Related to Polymer Properties and Structures to Deepen Your Understanding and Improve Your Mastery
