Photo AI
Last Updated Sep 24, 2025
Nylon and Polyester Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Nylon and Polyester quickly and effectively.
254+ students studying
Nylon and Polyester
Introduction
- Condensation polymers: Developed by the reaction of bifunctional monomers, resulting in extensive polymer chains accompanied by small molecules like water.
- These polymers are crucial in various industries, including textiles and engineering, due to their versatility, with applications ranging from clothing to packaging.
Condensation Polymers: Formation occurs through bifunctional monomer reactions, releasing small molecules such as water.
General Reaction Equation
- Monomer A–B combines with Monomer B–A, forming repeated units (-A–B-) within polymer chains.
- By-products: typically water.
Chemical Equation:
Differentiating Polymerisations
- Mono-functional monomers: Contain a single reactive group, utilised in addition polymerisation, generating no by-products.
- Bi-functional monomers: Essential for condensation polymerisation, featuring two reactive groups, yielding polymer chains and by-products such as water.
Differences
- Condensation polymerisation: Generates by-products through bifunctional monomers.
- Addition polymerisation: Produces no by-products and employs mono-functional monomers.
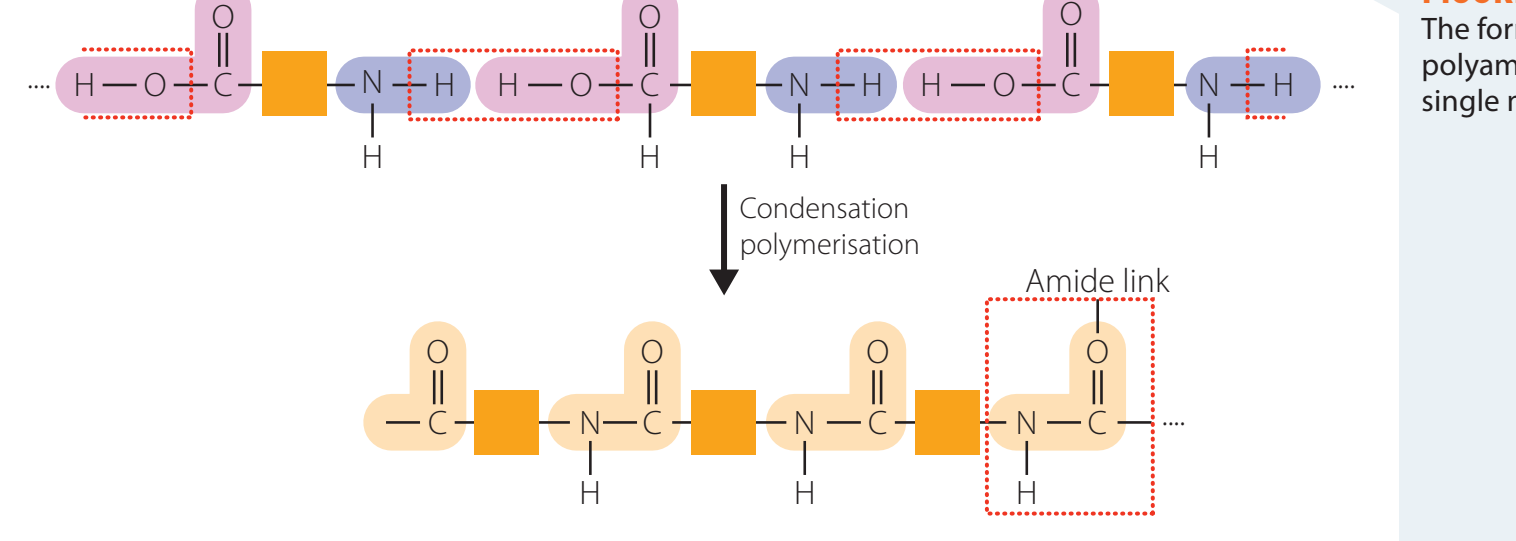
Overview of Nylon
Structure and Synthesis
-
Nylon: A class of polyamides recognised for its condensation polymer formation, durability, and flexibility.
-
Structure: Comprises repeating units with amide linkages (–CONH–), facilitating strong hydrogen bonds that enhance mechanical strength.
infoNoteAmide Linkages: Created between the carbonyl carbon of one molecule and the nitrogen of another, bolstering polymer strength.
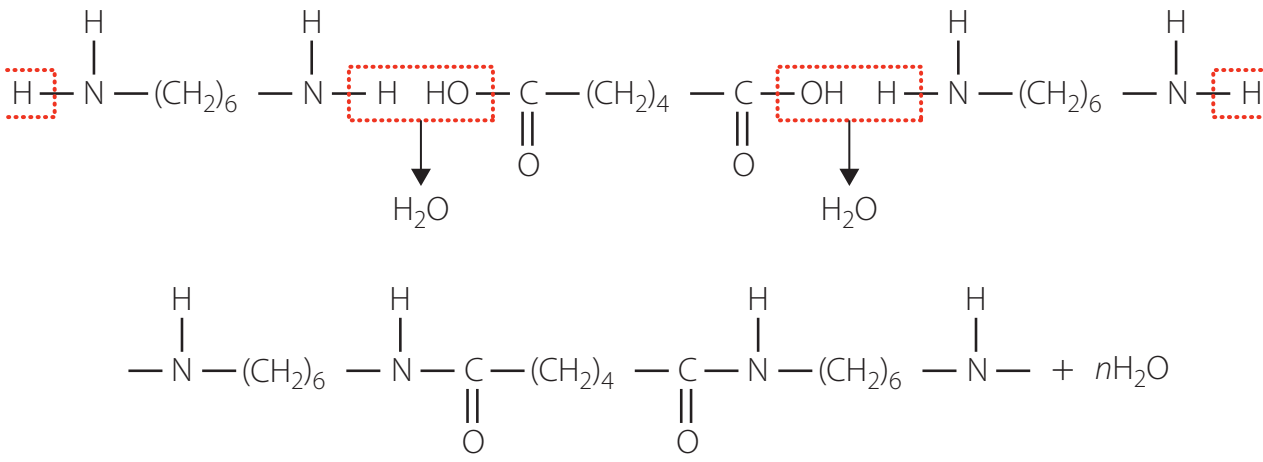
Synthesis Process
-
Monomers:
- Hexamethylene Diamine (C₆H₁₆N₂)
- Adipic Acid (C₆H₁₀O₄)
-
Temperature: 250-280°C, utilising a solvent system without a catalyst.
chatImportantAccurate temperature and solvent systems are vital for producing high-quality polymers.
Reaction
- Properties: Exhibits high tensile strength and temperature resistance.
Applications
- Used in engineering plastics, textiles, and automotive components.
- Environmental Impact: Non-biodegradable, presenting challenges for sustainable development.
Overview of Polyesters
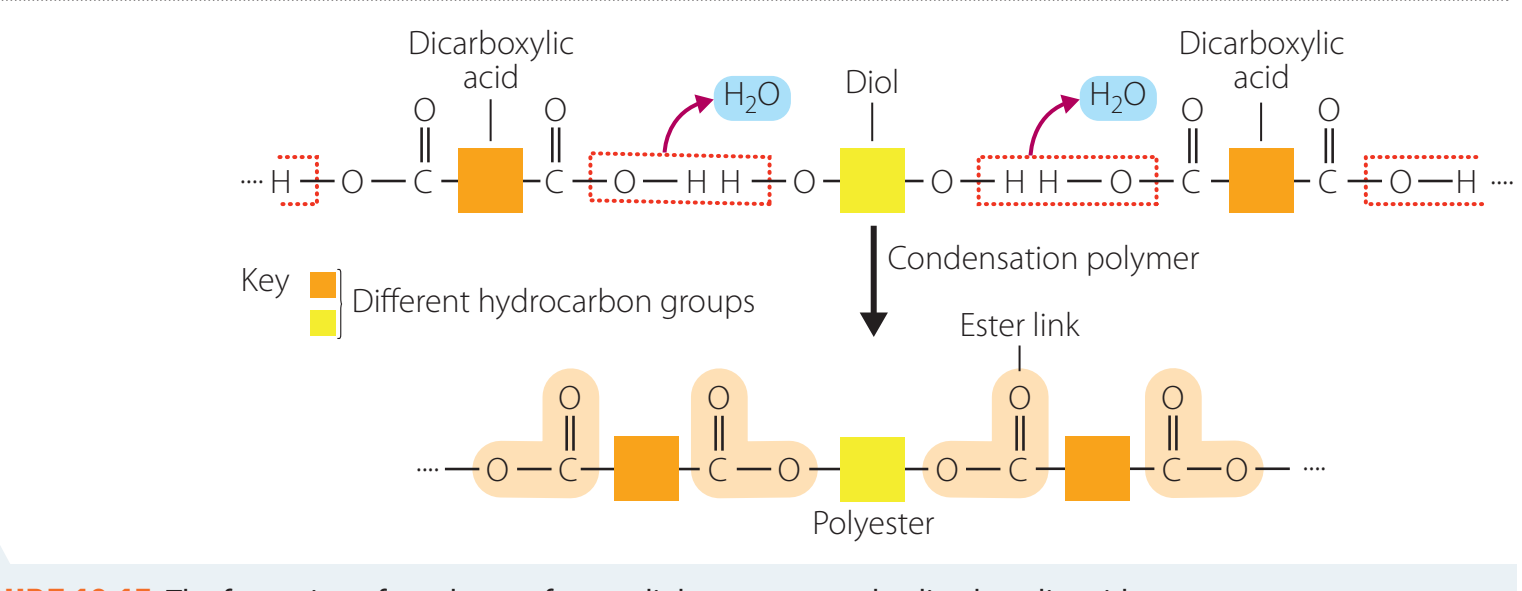
Introduction
- Polyesters: Produced through monomer interactions, including terephthalic acid and ethylene glycol.
- Use Cases: Common in textiles, packaging, and electronics.
Industrial Importance: Constitutes over 60% of global textile output.
Structure
- Polyethylene Terephthalate (PET) comprises terephthalate and ethylene glycol units.
- Properties: Semi-crystalline nature influences flexibility and strength.
Synthesis
- Reaction Equation:
- Catalyst: Antimony oxide is employed to accelerate the reaction rate.
Applications
- Packaging: Noted for recyclability and durability.
- Textiles: Chosen for wrinkle resistance and ease of dyeing.
Environmental Challenges and Recycling
- Production Issues: Heavily dependent on non-renewable resources and associated with emissions during production.
- Disposal: Frequently disposed of in landfills as non-biodegradable waste.
Recycling Importance: Critical in reducing carbon footprint and enhancing sustainability. Advances in recycling have elevated polyester as a sustainability leader.
Processes
- Mechanical Recycling: Involves physical techniques like shredding and remoulding.
- Chemical Recycling: Decomposes polymers into their original monomers.
| Material | Recycling Rate | Success Rate |
|---|---|---|
| Nylon | Low | Moderate |
| Polyester | Higher | High |
Biodegradable Alternatives
- Introduction of bio-based polymers, such as PLA, offers biodegradable solutions.
- Challenges: Faces high production costs comparable to organic products.
Summary Table
| Property | Nylon | Polyester |
|---|---|---|
| Elasticity | High | Moderate |
| Chemical Resistance | Excellent for oils and fuels | Moderate |
| Wear Resistance | High | Moderate |
| Weight | Light | Moderate |
| Thermal Stability | Excellent | Good |
Summary of Key Points
- Condensation polymers, essential to industrial applications, pose both challenges and solutions in advancing sustainability.
- Leveraging their characteristics effectively can promote eco-efficiency in modern material usage.
- Effective recycling and innovative advancements are crucial for mitigating environmental impacts.
Glossary:
- Crystallinity: Arrangement of molecules that affects strength and thermal resistance.
- Tensile Strength: Ability to withstand tension without breaking.
- Elasticity: Capacity of a material to resume its original shape post-deformation.

500K+ Students Use These Powerful Tools to Master Nylon and Polyester For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
188 flashcards
Flashcards on Nylon and Polyester
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards16 quizzes
Quizzes on Nylon and Polyester
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes27 questions
Exam questions on Nylon and Polyester
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Nylon and Polyester
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Nylon and Polyester
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Nylon and Polyester you should explore
Discover More Revision Notes Related to Nylon and Polyester to Deepen Your Understanding and Improve Your Mastery
