Photo AI
Last Updated Sep 24, 2025
Common Ion Effect Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Common Ion Effect quickly and effectively.
355+ students studying
Common Ion Effect
The Common Ion Effect is a significant principle in chemistry that describes the decrease in solubility of a compound when an ion that is already part of the compound is added to the solution. This phenomenon is important in various contexts, such as water treatment, pharmaceutical formulation, and industrial processes.
Introductory Overview
- Consider the issue of lead contamination in water systems. The common ion effect can address this by causing precipitations of harmful ions like lead, thus lowering their concentration.
- A comprehensive understanding of this effect is essential for both preventing undesirable chemical reactions and enhancing beneficial ones.
Le Chatelier's Principle
Explains how an equilibrium system adjusts to external changes to maintain stability.
Definition and Explanation
- Common Ion Effect: Decreased solubility of a compound in a solution when it contains an ion present in the compound.
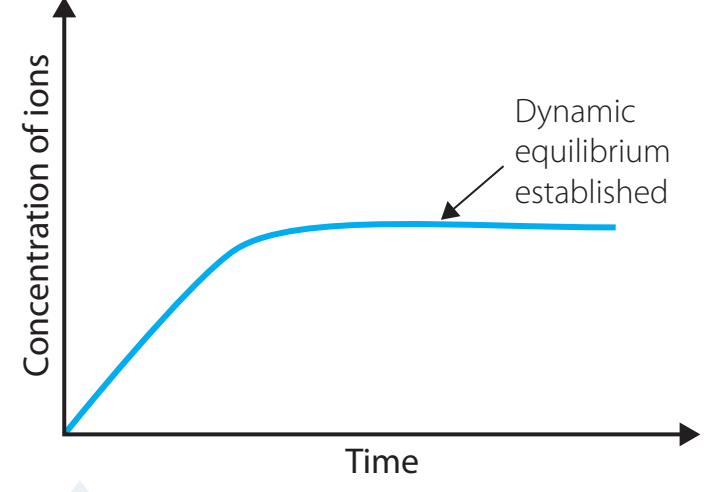
- Dynamic Equilibrium: Ongoing balance between dissolution and precipitation.
- Sparingly Soluble Salt: Compounds with minimal solubility in water.
- Solubility Product Constant (K): Indicates solubility under equilibrium conditions.
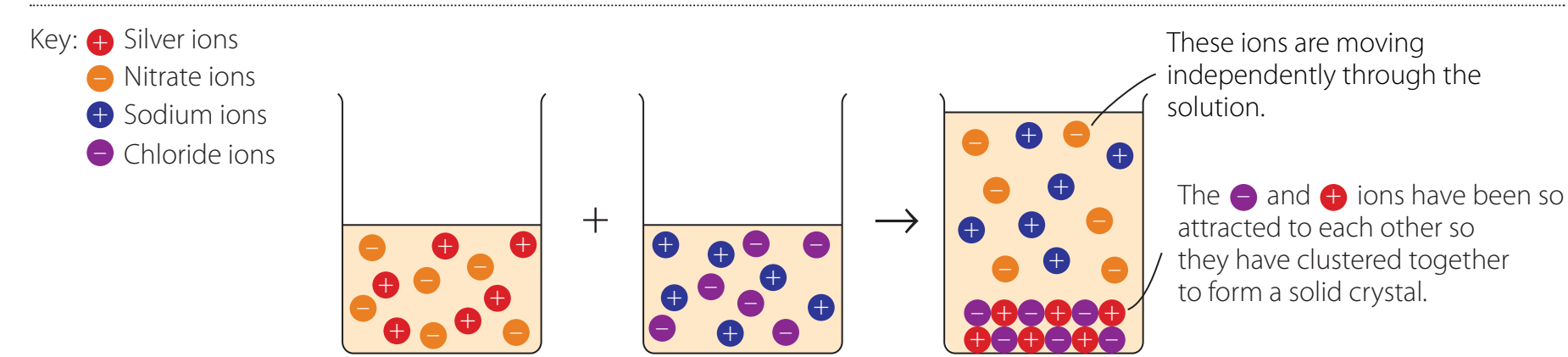
Real-world Application with Silver Chloride (AgCl)
- Equation:
- Practical Insight: Introducing table salt (NaCl) to a saltwater solution adds more Cl⁻ ions. This shifts the equilibrium to precipitate additional solid AgCl, thereby reducing its solubility.
- Visualisation: Particles continuously exchange between states, illustrating ongoing equilibrium.
Solubility Calculations with Common Ions
Overview
- The influence of common ions on solubility is measured by the solubility product constant (K), which is crucial in predicting solubility within solutions.
K Understanding
- Definition: The solubility product constant, K represents solute dissolution prior to precipitation.
Mathematical Framework
- K Formula:
- For a salt AB:
- For a salt AB:
- Denotes the ion concentrations, each raised to the power of their stoichiometric coefficients.
Example Problems
Example 1: Lead(II) Bromide
- Chemical Equilibrium:
- Common Ion Effect:
- Adding more bromide ions reduces the solubility of PbBr₂ by shifting the equilibrium to the left.
Example 2: Silver Chloride in Sodium Chloride Solution
- Influential Ion: Cl⁻ from NaCl affects the solubility of AgCl.
- K Expression:
- Step-by-Step Solution:
- Write the K expression: K = [Ag⁺][Cl⁻]
- If [Cl⁻] is known (e.g., from NaCl solution), rearrange to find [Ag⁺]: [Ag⁺] = K/[Cl⁻]
- The molar solubility of AgCl equals [Ag⁺] in this case
Practical Applications
Water Treatment
- Method: Introducing sodium chloride (NaCl) reduces calcium's solubility, prompting its precipitation.
- Outcome: Results in cleaner water, as depicted in the diagram.

Pharmaceutical Formulation
- Effect on Bioavailability: Common ions influence drug dissolution rates, affecting effectiveness.
- Example: Ibuprofen's bioavailability can be impacted by ions present in the stomach.

Environmental Implications
- Example: Discharge of ions by mining industries influences water solubility.
- Preventive Measures: Utilise alternative substances to decrease ion release.
Industrial Chemistry
- Process Optimisation: Controlling ion effects improves production efficiency and product quality.
- Case Study: The Bayer process demonstrates efficient alumina extraction.

Problem with Solution
Determine the solubility of AgCl in a 0.1 M NaCl solution, given K of AgCl = 1.8 × 10.
Solution:
-
Identify the equilibrium: AgCl(s) ⇌ Ag⁺(aq) + Cl⁻(aq)
-
Write the K expression: K = [Ag⁺][Cl⁻] = 1.8 × 10
-
Analyse the Cl⁻ concentration: [Cl⁻] = 0.1 M (from NaCl) + x (from AgCl dissolution) Since x will be very small compared to 0.1 M, we can approximate: [Cl⁻] ≈ 0.1 M
-
Calculate [Ag⁺]: [Ag⁺] = K/[Cl⁻] = (1.8 × 10)/(0.1) = 1.8 × 10 M
-
Determine solubility: The molar solubility of AgCl equals [Ag⁺] = 1.8 × 10 M
Therefore, the solubility of AgCl in 0.1 M NaCl is 1.8 × 10 M, significantly lower than its solubility in pure water (1.3 × 10 M), demonstrating the common ion effect.
Important Tips: Always account for all sources of common ions when assessing solubility changes.
500K+ Students Use These Powerful Tools to Master Common Ion Effect For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
242 flashcards
Flashcards on Common Ion Effect
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Common Ion Effect
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes20 questions
Exam questions on Common Ion Effect
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Common Ion Effect
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Common Ion Effect
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Common Ion Effect you should explore
Discover More Revision Notes Related to Common Ion Effect to Deepen Your Understanding and Improve Your Mastery
