Photo AI
Last Updated Sep 24, 2025
Solubility Rules Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Solubility Rules quickly and effectively.
355+ students studying
Solubility Rules
Introduction
Solubility equilibria: Describes the balance between a solid substance and its ions in a saturated solution.
Understanding solubility equilibria is essential for mastering chemical reactions, similar to how comprehending sugar's solubility affects sweetness.
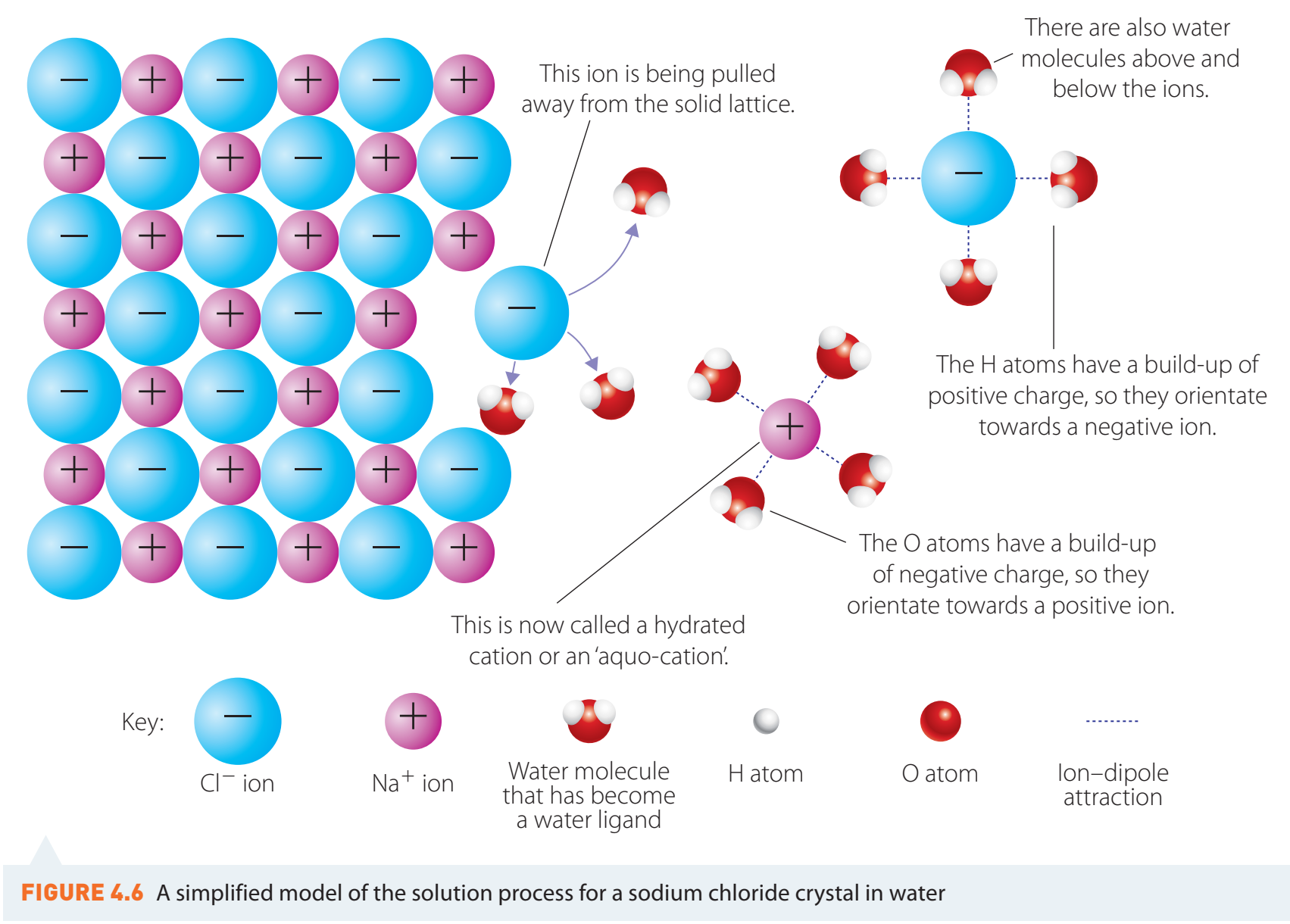
The dissolution of ionic compounds involves breaking a solid into individual ions in water. For instance, sodium chloride (NaCl) dissolves to form sodium (Na^+) and chloride (Cl^-) ions.
Solubility rules: Assist in predicting how ionic compounds behave in water—vital in fields like pharmaceuticals, where they impact drug effectiveness, and water treatment, where they aid in removing contaminants.
Solubility Rules: Play a significant role in predicting the water solubility of ionic compounds and understanding practical applications.
Key Concepts and Processes
Polarity of Water and Ion-Dipole Interactions
- Water Structure: Composed of two hydrogen atoms covalently bonded to one oxygen atom.
- Charge Distribution:
- Oxygen carries a partial negative charge.
- Hydrogen carries a partial positive charge.
- Polarity: The unequal charge distribution facilitates effective dissolution.
Polarity: Unequal electric charge distribution within a molecule, critical for interactions with ionic compounds.
- Ion-Dipole Interactions: Water's polar nature allows it to orient its partial charges towards ions—oxygen aligns with cations, hydrogen with anions—facilitating the breakdown of ionic crystal lattices.
Ion-Dipole Interactions: The attractions between ion charges and polar molecules promote dissolution.
Energy Considerations
- Hydration Energy: Released when ions are surrounded by water molecules.
- Lattice Energy: Required to break ions apart from lattice structures.
If hydration energy exceeds lattice energy, dissolution becomes feasible.
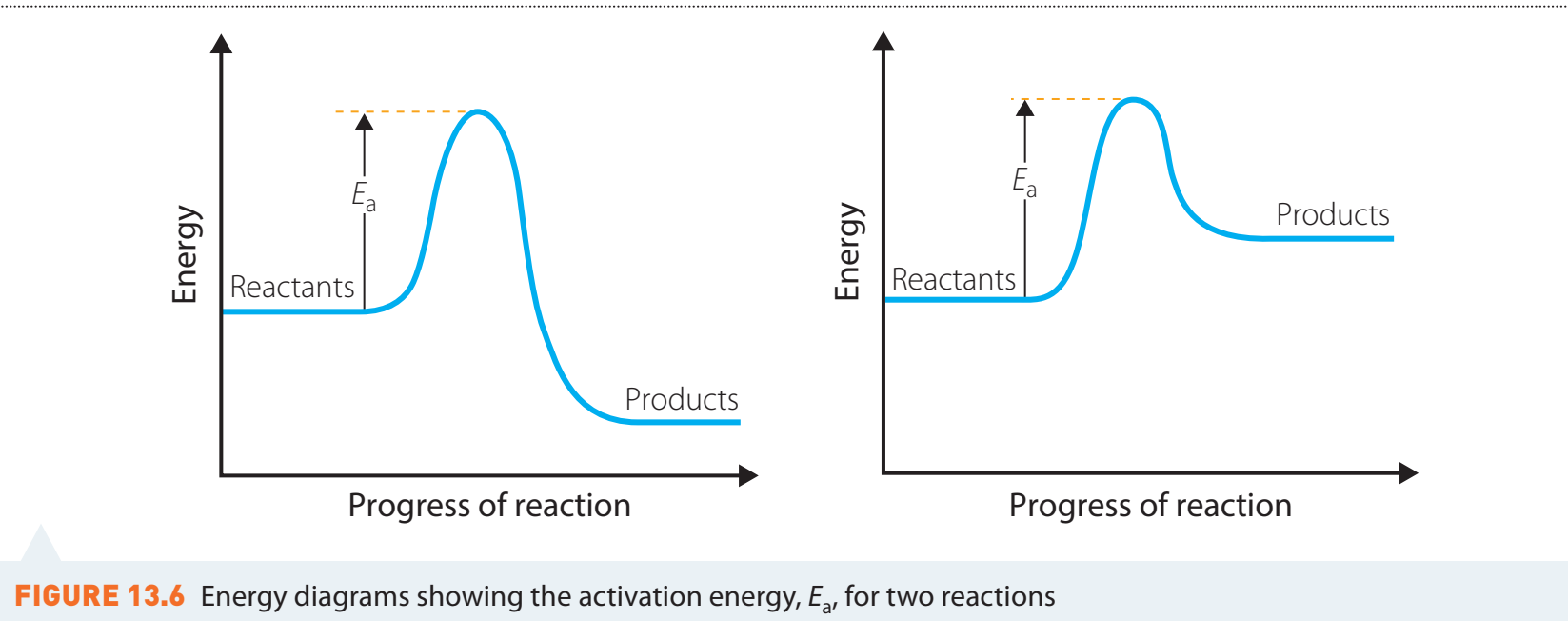
Temperature's Impact on Dissolution
-
Dissolution Rate: Increasing temperature enhances molecular motion, accelerating dissolution, similar to how sugar dissolves faster in hot tea.
-
Equilibrium Solubility:
- Endothermic Processes: Solubility increases with temperature.
- Exothermic Processes: Solubility may decrease as temperature rises.
Dissociation of Ionic Compounds
When NaCl dissolves, it dissociates into ions:
Common Ion Effect
- Effect: Introducing a common ion decreases a compound's solubility.
- Example: Adding NaCl to an AgCl solution reduces its solubility following Le Chatelier's principle.
Understanding the common ion effect is crucial for predicting chemical outcomes.

Case Study: Cycad Fruit Detoxification
- Detoxification Process:
- Applying solubility principles to dissolve and eliminate toxins.
- Soaking: Submerging fruits in water to dissolve toxins.
- Rinsing: Repeatedly done to ensure safety for consumption.

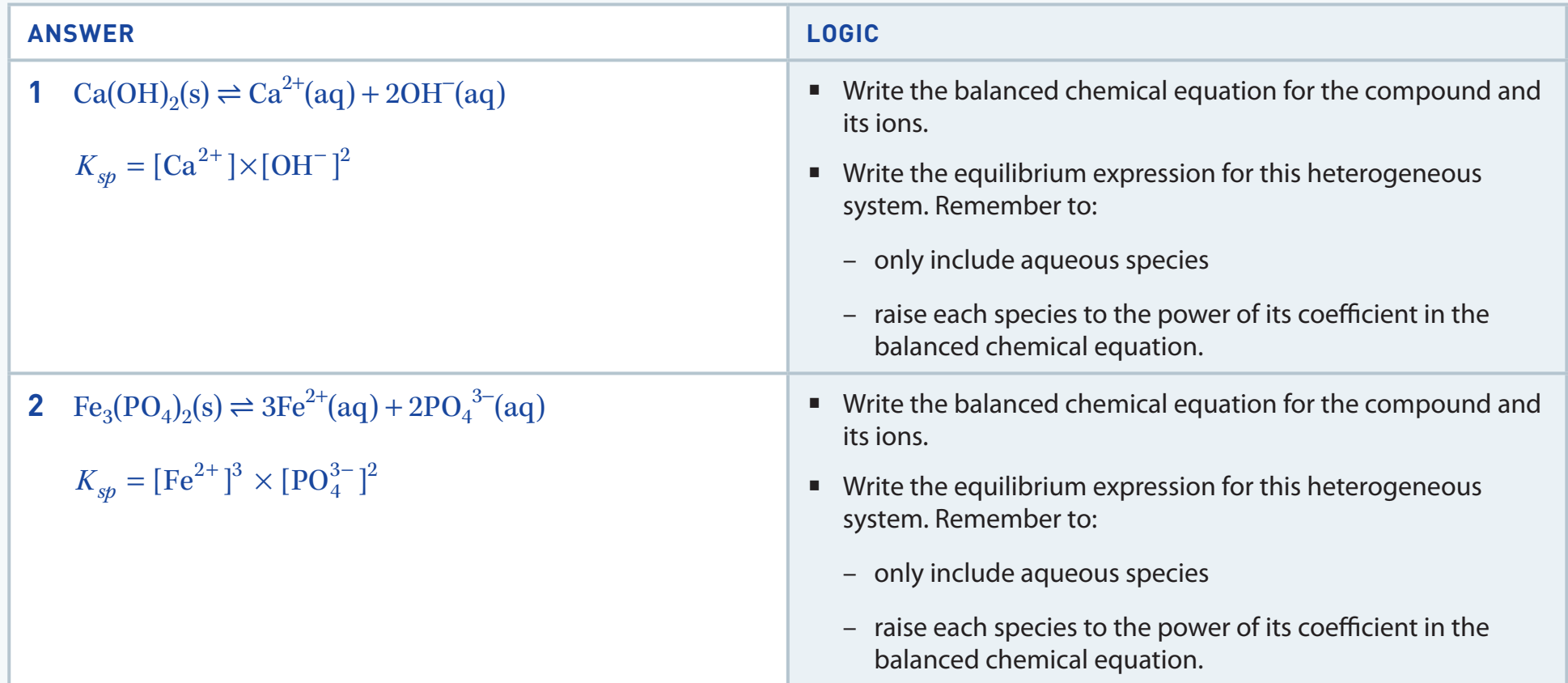
Solubility Product Concept (Ksp)
- Ksp: Used to predict when a precipitate will form as ions reach saturation.
Understanding Ksp is vital in pharmaceutical contexts for drug formulation.
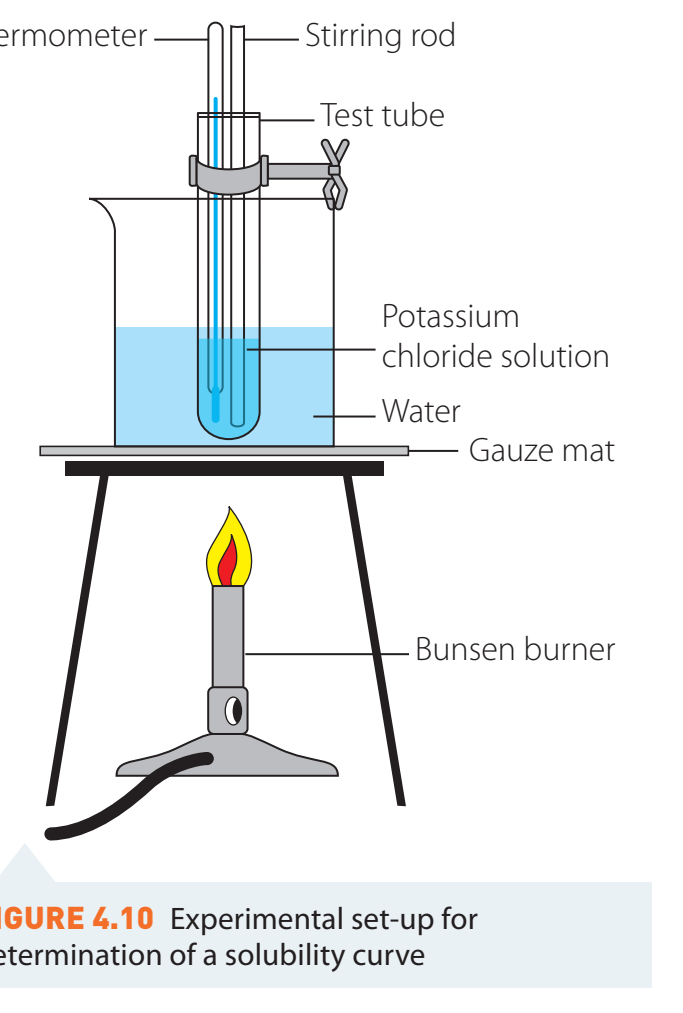
Laboratory Experiment Setup
Equipment and Chemicals
- Equipment: Beakers, test tubes, pipettes.
- Chemicals: Potassium chloride, silver nitrate.
Safety Precautions
- Safety Gear: Wear gloves and goggles.
- Hazard Prevention: Utilise fume hoods for handling volatile compounds.
Misconceptions About Solubility
Misconception 1: All Ionic Compounds Are Soluble
- Insoluble Examples:
- Barium sulphate (BaSO₄): Has low solubility due to its high lattice energy.
- Lead chloride (PbCl₂): Limited solubility due to strong ionic bonds.
Misconception 2: Equilibrium as Static State
- Equilibrium involves dynamic processes.
Cultural and Scientific Insights
- Traditional practices emphasise the sustainable management of resources and community health.

Practical Implications
- Discussing adherence or deviation from rules impacts experimental outcomes.
- Explore common ion effects in real-world scenarios like environmental engineering and healthcare.
Worked Example
Question: Calculate the solubility of calcium fluoride (CaF₂) in water if its Ksp = 3.9 × 10^-11.
Solution:
-
Write the dissociation equation:
-
Define the Ksp expression:
-
Let's call the solubility of CaF₂ as s mol/L.
- Then [Ca²⁺] = s
- And [F⁻] = 2s (since each formula unit provides 2 F⁻ ions)
-
Substitute into the Ksp expression:
-
Solve for s:
Therefore, the solubility of CaF₂ in water is 2.1 × 10^-4 mol/L.
Practice Questions with Solutions
-
Question: Predict whether a precipitate will form when 75.0 mL of 0.0050 M lead(II) nitrate is mixed with 125.0 mL of 0.0100 M sodium chloride. (Ksp for PbCl₂ = 1.6 × 10^-5)
Solution: First, calculate the concentrations after mixing:
Total volume = 75.0 mL + 125.0 mL = 200.0 mL
[Pb²⁺] = (0.0050 M × 75.0 mL) ÷ 200.0 mL = 1.88 × 10^-3 M
[Cl⁻] = (0.0100 M × 125.0 mL) ÷ 200.0 mL = 6.25 × 10^-3 M
For PbCl₂, Q = [Pb²⁺][Cl⁻]² = (1.88 × 10^-3)(6.25 × 10^-3)² = 7.34 × 10^-8
Since Q > Ksp (7.34 × 10^-8 > 1.6 × 10^-5), a precipitate will form.
-
Question: The solubility of silver chloride (AgCl) in pure water is 1.3 × 10^-5 mol/L. Calculate its Ksp value.
Solution: For AgCl → Ag⁺ + Cl⁻
[Ag⁺] = [Cl⁻] = 1.3 × 10^-5 mol/L
Ksp = [Ag⁺][Cl⁻] = (1.3 × 10^-5)(1.3 × 10^-5) = 1.7 × 10^-10
500K+ Students Use These Powerful Tools to Master Solubility Rules For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
242 flashcards
Flashcards on Solubility Rules
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Solubility Rules
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes20 questions
Exam questions on Solubility Rules
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Solubility Rules
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Solubility Rules
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Solubility Rules you should explore
Discover More Revision Notes Related to Solubility Rules to Deepen Your Understanding and Improve Your Mastery
