Photo AI
Last Updated Sep 24, 2025
Measuring Solubility Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Measuring Solubility quickly and effectively.
237+ students studying
Measuring Solubility
Solution equilibria involve the balance between dissolved ions in a saturated solution. Understanding these principles is critical for mastering the behaviour of chemical solutions.
Introduction
- Saturated Solutions: Solutions that contain the maximum concentration of ions at a given temperature.
- Ionic Compounds: Consist of charged ions.
- Example: Table salt (NaCl).
- Solubility Product (): Indicates the maximum solubility level of ionic compounds. Knowing is essential to predict the solubility of compounds.
Key Terms
- Saturated Solutions: Maximum ion concentration in solutions.
- Ionic Compounds: Charged ions held together by electrostatic forces.
- Solubility Product (): Determines solubility.
Key Concepts
-
Dynamic Equilibrium:
- Occurs when the rate of dissolution equals the rate of precipitation.
- Maintains a stable concentration of ions.
-
Solubility Product ():
- An equilibrium constant that indicates solubility level.
- A higher implies greater solubility.
-
Heterogeneous Equilibrium:
- Reactions involving different phases, such as solid and liquid.
Visual Illustrations
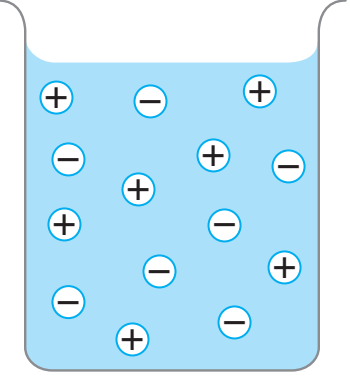
- Diagram Explanation: Displays ion balance in the solution.
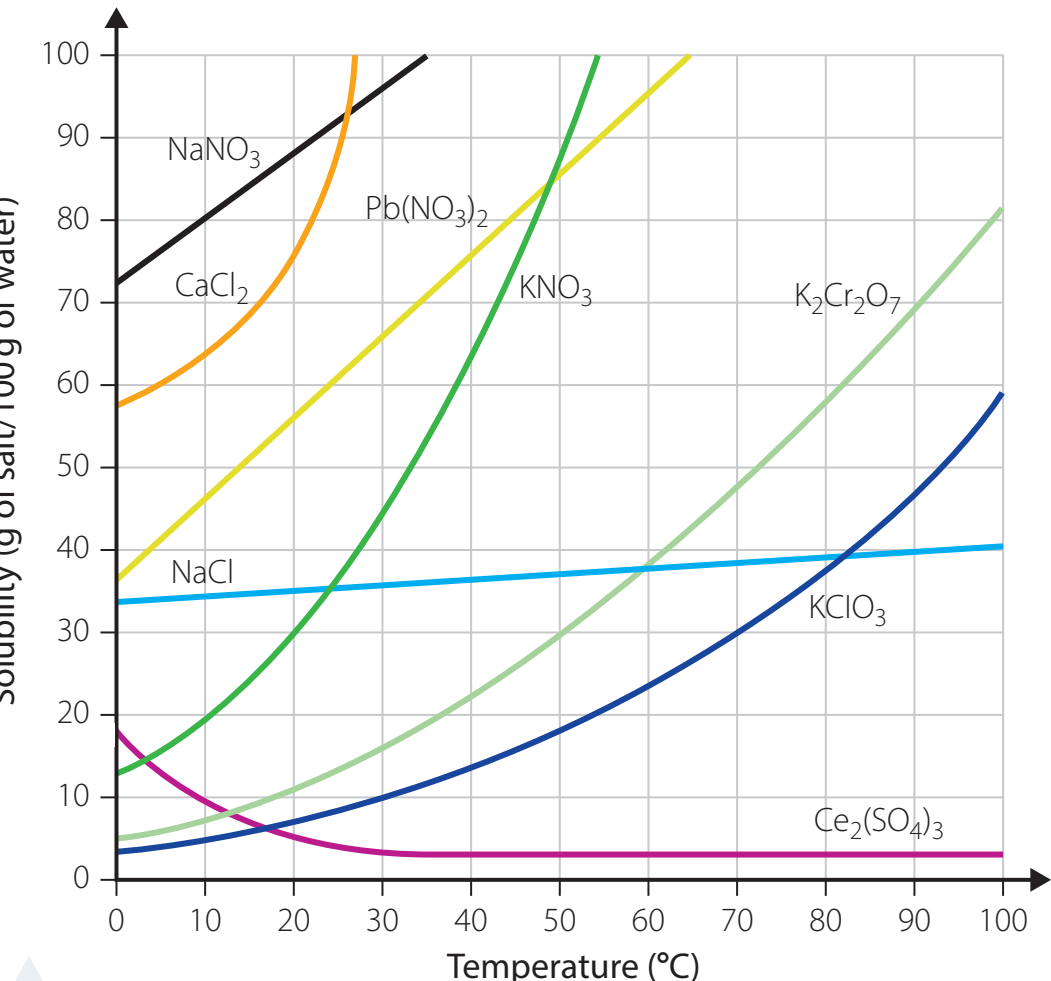
- Solubility Curve Explanation: Illustrates the temperature dependence of solubility.
Steps to Derive Equilibrium Expressions
1. Identify the Dissociation Reaction
- Dissociation Equation Example:
- Note: Only dissolved ions appear in expressions.
Ensure dissociation equations and express stoichiometry correctly.
2. Expression Formation Using
-
Expression Format:
- General Formula:
- General Formula:
-
Example Using Silver Chloride:
- Dissociation:
- Ksp Expression:
Quick Tip: Solids and liquids are excluded from expressions.
Solubility and Calculations
Step-by-Step Calculation Example Using :
-
Write Dissociation Equation:
-
Formulate :
-
Calculate Solubility:
- If we define solubility as mol/L, then
- Therefore:
-
Solving for (Given ):
Challenges and Strategies
Understanding and
- Key Conditions:
-
Unsaturated:
-
Saturated at equilibrium:
-
Supersaturated:
-
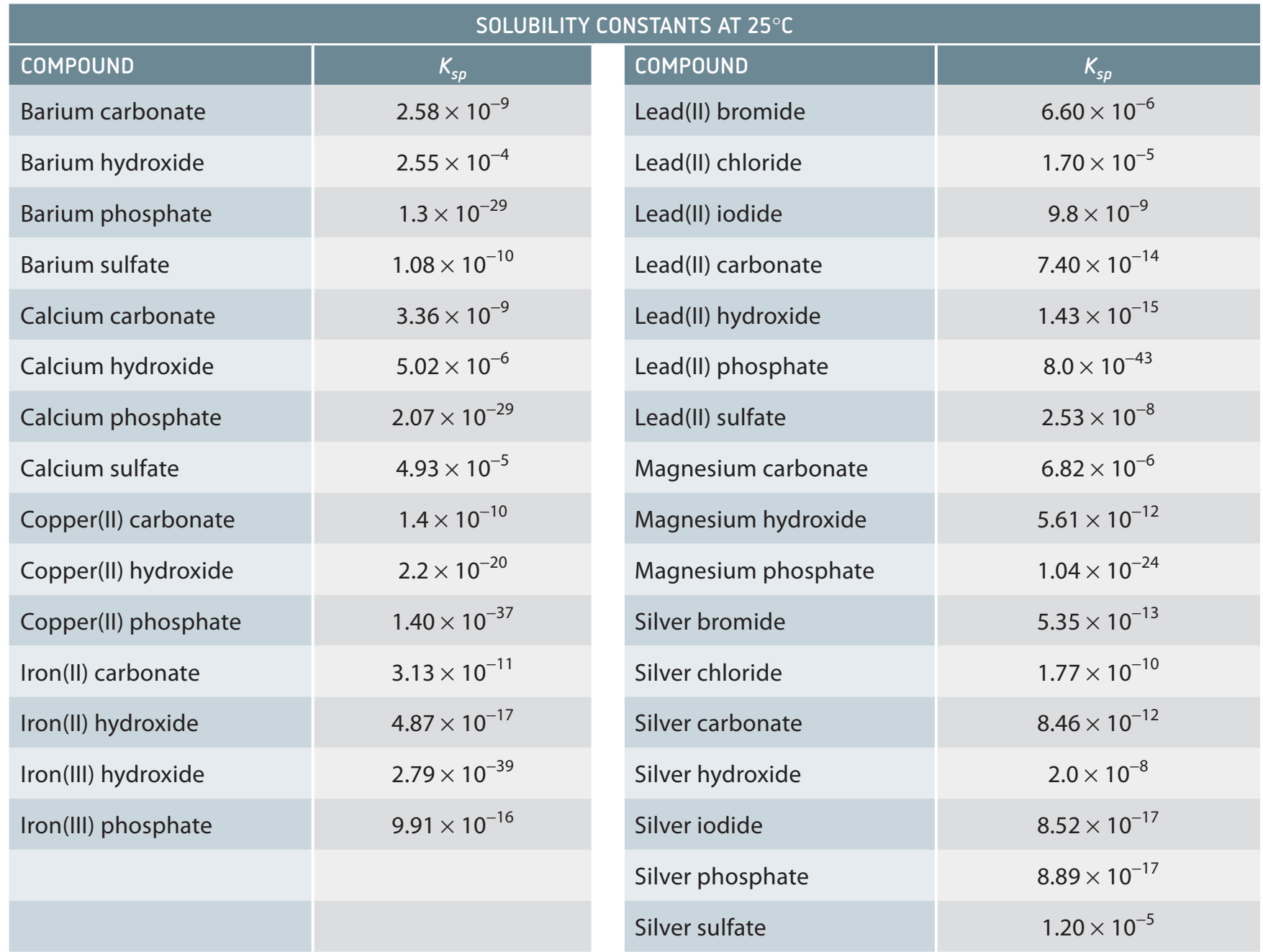
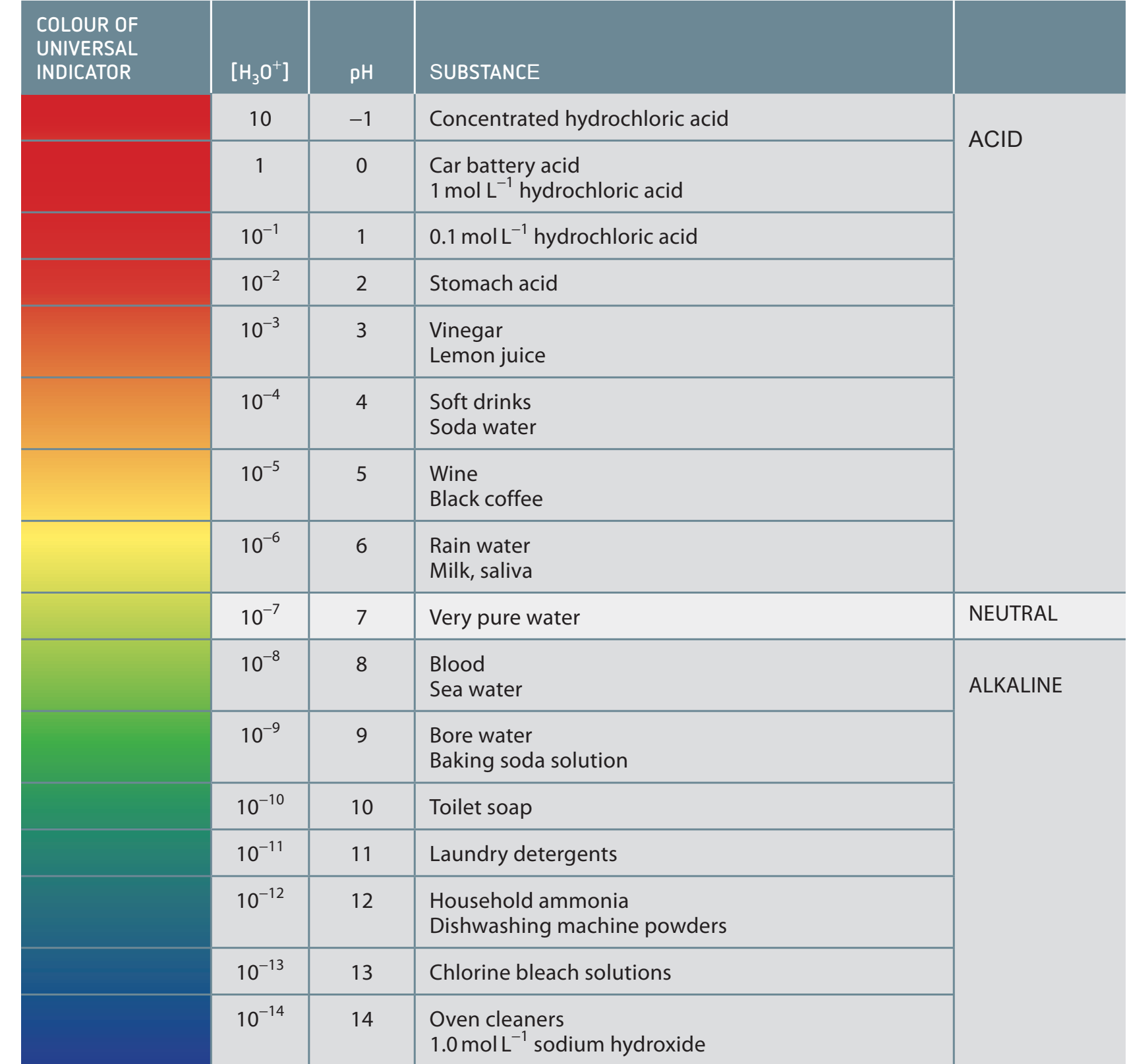
pH and Common Ion Effect
- pH Impact: Influences solubility, with calcium carbonate dissolving more as pH decreases.
Exam Tips
- Practise with various ionic compounds.
- Verify understanding of stoichiometry and .
- Regularly solve problems to reinforce concepts.
500K+ Students Use These Powerful Tools to Master Measuring Solubility For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
242 flashcards
Flashcards on Measuring Solubility
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Measuring Solubility
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes20 questions
Exam questions on Measuring Solubility
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Measuring Solubility
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Measuring Solubility
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Measuring Solubility you should explore
Discover More Revision Notes Related to Measuring Solubility to Deepen Your Understanding and Improve Your Mastery
