Photo AI
Last Updated Sep 24, 2025
Ksp and Solubility Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Ksp and Solubility quickly and effectively.
236+ students studying
Ksp and Solubility
Introduction
Calculating solubility using the solubility product constant (Ksp) is vital in areas such as environmental science and industrial chemistry. This measurement helps determine water quality and affects processes, including mineral extraction.
Concept of Equilibrium in Saturated Solutions
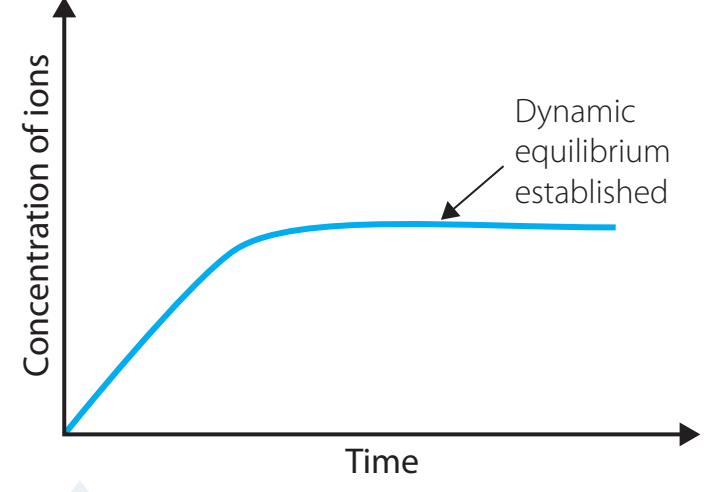
- Chemical Equilibrium: A dynamic condition where the rate at which the solute dissolves equals the rate of precipitation in a saturated solution.
- Saturated Solutions: These occur when no additional solute can dissolve under specific conditions, such as temperature and pressure.
- Dynamic Equilibrium: In this state, solute particles dissolve and precipitate at equal rates, maintaining a stable composition.
- Examples:
- NaCl dissolving in water.
- Chalk (calcium carbonate) in water, showcasing limited solubility.
Saturated Solution: A solution where the maximum amount of solute has been dissolved.
Diagram: Illustrating Dynamic Equilibrium
Solubility Product Constant (Ksp)
- Ksp: The equilibrium constant for saturated solutions, measuring the maximum potential ion concentration.
Deriving the Ksp Expression
- Consider a generic ionic compound AB dissolving into A⁺ and B⁻. The Ksp is represented as:
Example Derivation – Lead Chloride (PbCl₂)
- Equilibrium Equation:
- PbCl₂(s) ⇌ Pb²⁺(aq) + 2Cl⁻(aq)
- Ksp Expression:
- .
- Significance: The Ksp indicates the ion concentration in a solution.
Ksp: Equilibrium constant measuring maximum potential ion concentration.
Factors Affecting Solubility
Introduction to Factors Affecting Solubility
- Solubility: The capability of a solute to dissolve in a solvent, indicating how much solute can dissolve before the solution becomes saturated.
- Key Influences:
- Enthalpy (ΔH) and Entropy (ΔS): Energy changes and disorder impacting solubility.
- Polarity: The principle of 'like dissolves like.'
- Hydration Energy: The energy released when ions are surrounded by water molecules.
Solvent and Solute Nature
- Hydration Spheres: Form around ions, enhancing solubility by stabilising dispersed ions.
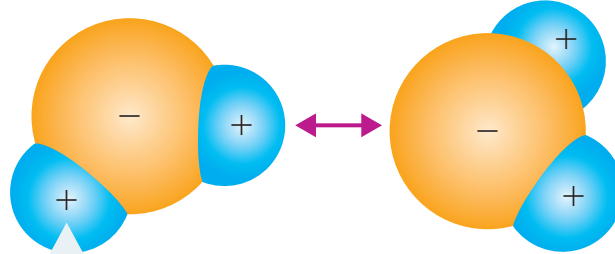
Ion Separation and Energy Exchange
- Lattice Energy: The energy required to separate ions in a crystal lattice. Balancing with Hydration Energy influences solubility.
Compare the solubility of NaCl and MgO to understand energy effects.
Temperature Effects
-
Effect of Temperature:
- Endothermic reactions benefit from increased temperature.
- Exothermic reactions may decrease in solubility with heat.
-
Illustrated example: More sugar dissolves in hot versus cold tea.
-
Van't Hoff Equation:
Common Ion Effect
-
Common Ion Effect: Solubility reduction occurs when a solution already contains a common ion.
-
Example: Adding NaCl to a solution of AgCl decreases AgCl's solubility due to the shared chloride ion.

Recognise and anticipate common ion effects.
Solubility Product Constant (Ksp) vs. Reaction Quotient (Q)
- Ksp: A threshold indicating saturation of ions in a solution.
- Q (Reaction Quotient): Measures the current ion product, compared to Ksp, to assess precipitation likelihood.
Decision-Making Procedure
- Calculate Q using initial ion concentrations and compare to Ksp:
- Q < Ksp: Undersaturated (no precipitation).
- Q = Ksp: Saturated equilibrium (no additional precipitate).
- Q > Ksp: Precipitation occurs.
The role of Q is crucial for comparing current ion conditions to Ksp, guiding precipitation likelihood.
Essential Formulas for Ksp-related Calculations
- Ksp Expression: Defined by:
- Describes equilibrium in a saturated solution like .
Practice Problems
-
Example 1: Calculate solubility for given :
- Equation:
- Ksp Expression:
- Solving for : mol/L
-
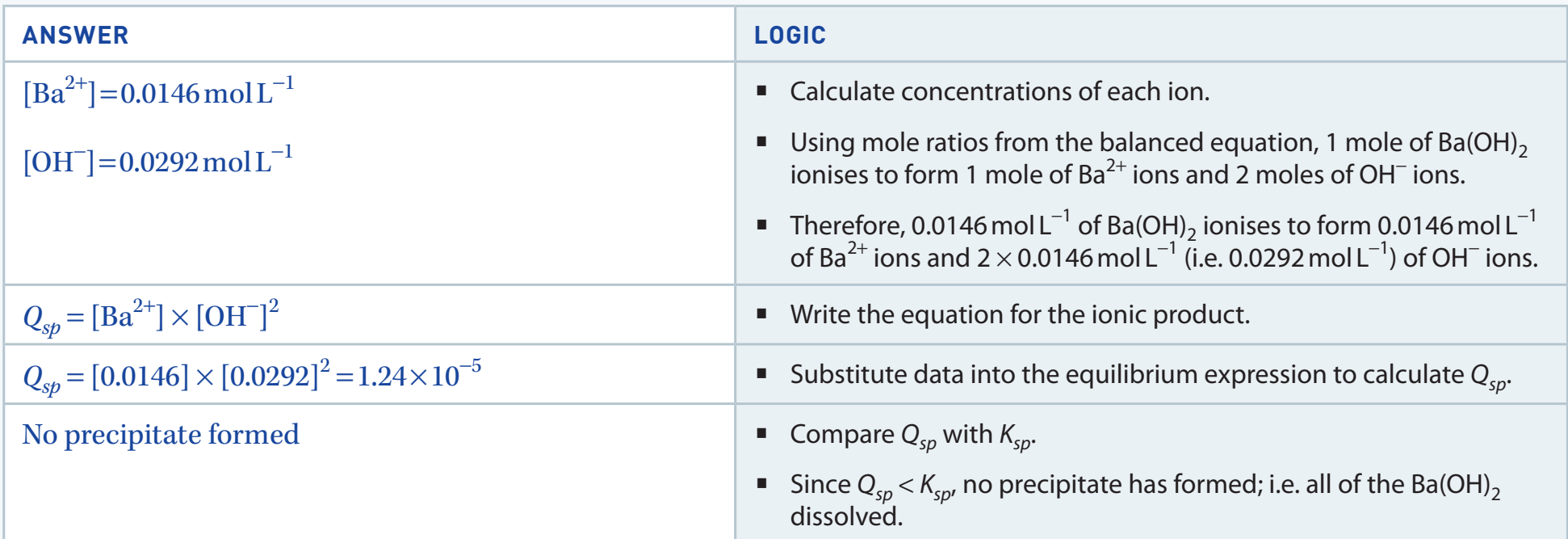
Problem 2: Calculate the molar solubility of BaSO₄ in a 0.05 M solution of Na₂SO₄ given Ksp = 1.1 × 10⁻¹⁰.
- Equation:
- Initial = 0.05 M (from Na₂SO₄)
- Let = molar solubility of BaSO₄
- At equilibrium: and
- Since will be very small compared to 0.05:
- mol/L
Exam Tips
- Emphasise the comparison between Q and Ksp in assessing potential precipitate formation.
- Consider temperature's effect on Ksp during calculations.
Ensure correct stoichiometric calculations with multi-ion compounds.
Breaking down equilibrium expressions, stoichiometry, and handling ion effects will enhance problem-solving skills.
500K+ Students Use These Powerful Tools to Master Ksp and Solubility For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
242 flashcards
Flashcards on Ksp and Solubility
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards21 quizzes
Quizzes on Ksp and Solubility
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes20 questions
Exam questions on Ksp and Solubility
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Ksp and Solubility
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Ksp and Solubility
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Ksp and Solubility you should explore
Discover More Revision Notes Related to Ksp and Solubility to Deepen Your Understanding and Improve Your Mastery
