Photo AI
Last Updated Sep 24, 2025
Acid-Base Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Acid-Base Reactions quickly and effectively.
206+ students studying
Acid-Base Reactions
Acid-base reactions: Acids are substances that increase the concentration of H⁺ ions in solution, while bases increase the concentration of OH⁻ ions. These reactions are vital in various everyday and industrial processes and constitute a fundamental component of chemical theory.
Introduction
- Balanced chemical equations illustrate the law of conservation of mass, essential for predicting chemical reaction outcomes and ensuring safety in laboratory environments.
- Significance: Acid-base reactions are pivotal in academia and industry, particularly in fields such as pharmaceuticals and agriculture.
Definitions and Distinctions
-
Acid: A substance that increases the concentration of H⁺ ions in solution. According to Arrhenius, acids release hydrogen ions (H⁺) when dissolved in water, thereby increasing acidity.
- Example: Hydrochloric acid (HCl) facilitates digestion.
-
Base: A substance that increases OH⁻ ion concentration in solution. Bases release hydroxide ions in aqueous solutions.
- Example: Sodium hydroxide (NaOH) is commonly used in cleaning solutions.
-
Brønsted-Lowry Theory:
- Acid: Proton donor
- Base: Proton acceptor
- Example: Ammonia (NH₃) accepts H⁺ to form ammonium (NH₄⁺).
Exothermic Reaction: A chemical reaction that releases heat, causing an increase in the surrounding temperature.
Reactions Involving Acids and Bases
Neutralisation Reactions
- Neutralisation produces water and a salt as products.
- Example Reaction:
- Step-by-Step:
- HCl dissociates into H⁺ and Cl⁻.
- NaOH dissociates into Na⁺ and OH⁻.
- H⁺ combines with OH⁻ to form water (H₂O).
- Na⁺ and Cl⁻ form NaCl (table salt).
- Step-by-Step:
Acid-Metal Reactions
- Example Reaction:
- Step-by-Step:
- Zinc reacts with HCl, displacing H⁺ ions.
- H⁺ ions combine to form H₂ gas, observable as bubbles.
- Step-by-Step:
These reactions are applied practically, such as in antacid medications and preventing material corrosion.
Historical Development of Acid-Base Theories
-
Arrhenius Model: Acids generate H⁺ ions in water; bases produce OH⁻ ions.
-
Brønsted-Lowry Model: Broadens the definition of acids and bases to non-aqueous solutions.
-
Lewis Theory: Focuses on electron pair donation and reception.
-
Comparison Table: Differentiates Arrhenius, Brønsted-Lowry, and Lewis theories, highlighting the differences between electron and proton transfer and the roles of solvents.
Misunderstandings and Practical Usage
Distinguishing between proton and electron transfers is crucial. Accurate application of theories is essential for understanding processes like biochemical pathways.
- Example in Practice: Catalyst use in renewable biofuel production relies on optimising acid-base reactions.
Nomenclature and Properties of Inorganic Acids and Bases
IUPAC Nomenclature
- Oxoacids: Contain oxygen. Example: H₂SO₄ - Sulphuric Acid.
- Binary Acids: Prefixed with 'hydro-', e.g., HCl - Hydrochloric Acid.
- Bases: Named by the metal followed by 'hydroxide', e.g., NaOH - Sodium Hydroxide.
pH Levels: Acids have a pH < 7; bases have a pH > 7.
- Conductivity: Strong electrolytes dissociate completely, whereas weak ones do so partially.
- Reactivity: Acid-metal interactions release hydrogen gas.
Preparation and Use of Indicators
Key Concepts
Indicators are substances that change colour in response to pH changes.
-
Types of Indicators:
- Litmus: Red in acidic solutions, blue in basic solutions.
- Phenolphthalein: Colourless in acidic conditions, turns pink in basic solutions.
-
Practical Experiments: Use natural indicators, such as red cabbage, to observe colour changes in solutions with varying pH levels.
Working with Equations and Calculations
Writing and Balancing Equations
- Steps: Determine reactants and products, confirm formulas, write, and balance the chemical equations.
Examples and Exercises
-
Example: Neutralise HCl with NaOH and balance the equation. This balanced equation shows that one mole of HCl reacts with one mole of NaOH to produce one mole of NaCl and one mole of water.
-
Practice: Write and balance: Solution: Here, two moles of HCl react with two moles of sodium to produce two moles of sodium chloride and one mole of hydrogen gas.
Neutralisation and Enthalpy
Thermochemistry: Neutralisation reactions are exothermic, with heat released measurable using calorimetric methods.
Hess's Law: Demonstrates that enthalpy changes are consistent regardless of the reaction pathway taken.
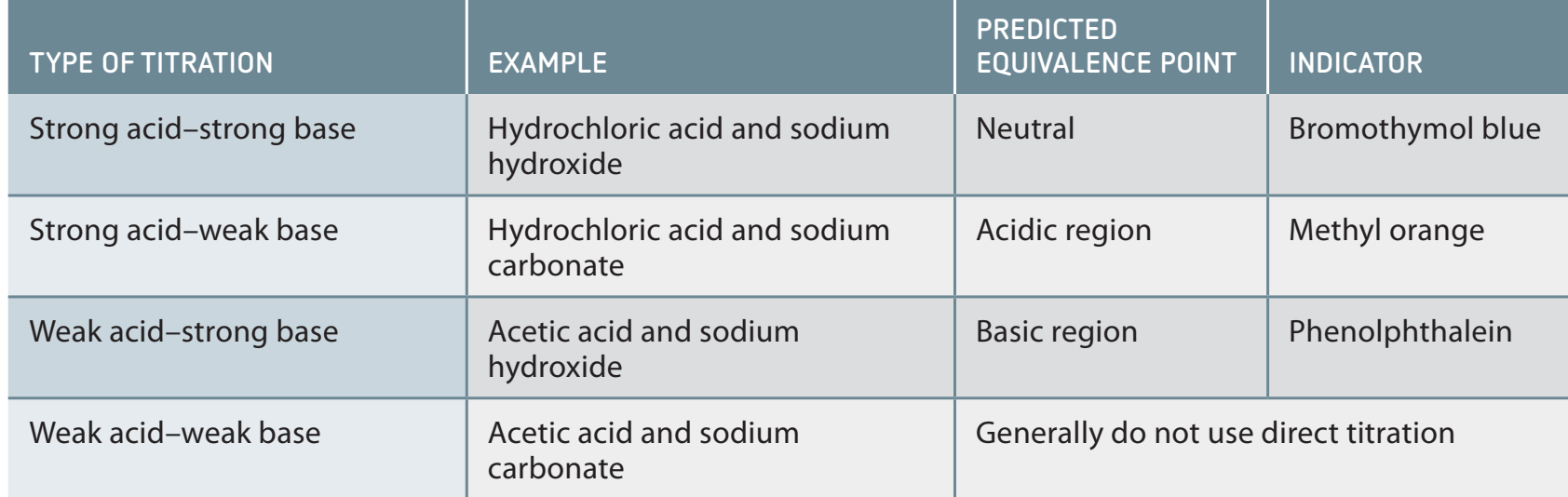
Titrations and Buffers
Titrations
- Used to determine the concentration of a solution with precision, observing the endpoint through a colour change.
- Key Concepts: Titrant versus analyte, and understanding the equivalence point.
Buffers
- Help maintain pH stability, crucial in both biological and chemical systems.
- Employ the Henderson-Hasselbalch equation for effective calculations.
Overview of Practice Applications
-
Industrial Use: Lime can neutralise soil acidity, and acetate is used in pharmaceutical synthesis.
-
Environmental Concerns: Lime treatment helps mitigate acid rain, supporting ecosystem protection.
-
Practical Exploration: Engage in simple experiments like testing household solutions with pH indicators.
500K+ Students Use These Powerful Tools to Master Acid-Base Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
209 flashcards
Flashcards on Acid-Base Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Acid-Base Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes23 questions
Exam questions on Acid-Base Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Acid-Base Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Acid-Base Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Acid-Base Reactions you should explore
Discover More Revision Notes Related to Acid-Base Reactions to Deepen Your Understanding and Improve Your Mastery
