Photo AI
Last Updated Sep 24, 2025
Precipitation Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Precipitation Reactions quickly and effectively.
362+ students studying
Precipitation Reactions
Introduction to Precipitation Reactions
- Precipitation Reaction: A chemical process where two soluble salts in a solution combine to form an insoluble solid, known as a precipitate.
- Precipitate: A solid that forms within a liquid solution and settles out at the bottom.
- Soluble: The capacity of a substance to dissolve within a liquid.
- Precipitate: A solid that emerges and settles out from a liquid mixture.
- Soluble: Capable of dissolving in a liquid.
Importance
- Precipitation reactions are essential in fields such as environmental science, medicine, and industry. They are utilised to:
- Separate mixtures.
- Purify compounds.
- Treat wastewater.
Solubility Rules and Prediction
Solubility: The property that enables a substance to dissolve in a solvent, essential for predicting chemical reactions.
Detailed Solubility Rules Table
- Nitrates (NO₃⁻): Soluble without any exceptions.
- Chlorides (Cl⁻): Soluble except when combined with Ag⁺ and Pb²⁺.
- Sulfates (SO₄²⁻): Soluble, except with Ba²⁺, Pb²⁺, or Ca²⁺.
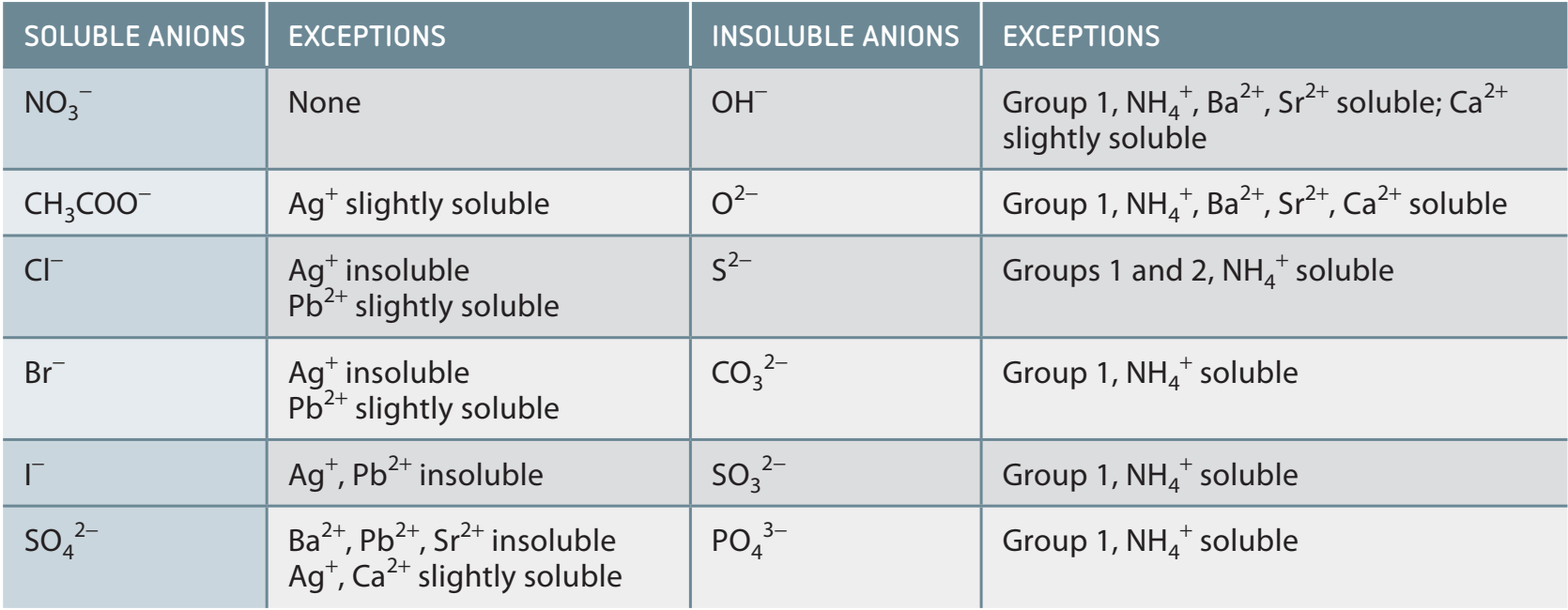
Avoid common errors: Be aware of exceptions!
Example Illustration
Key Experiment:
- Reactants: Barium chloride (BaCl₂) and sodium sulfate (Na₂SO₄).
- Products: Barium sulfate (BaSO₄, precipitate) and sodium chloride (NaCl).
- Reaction Equation:
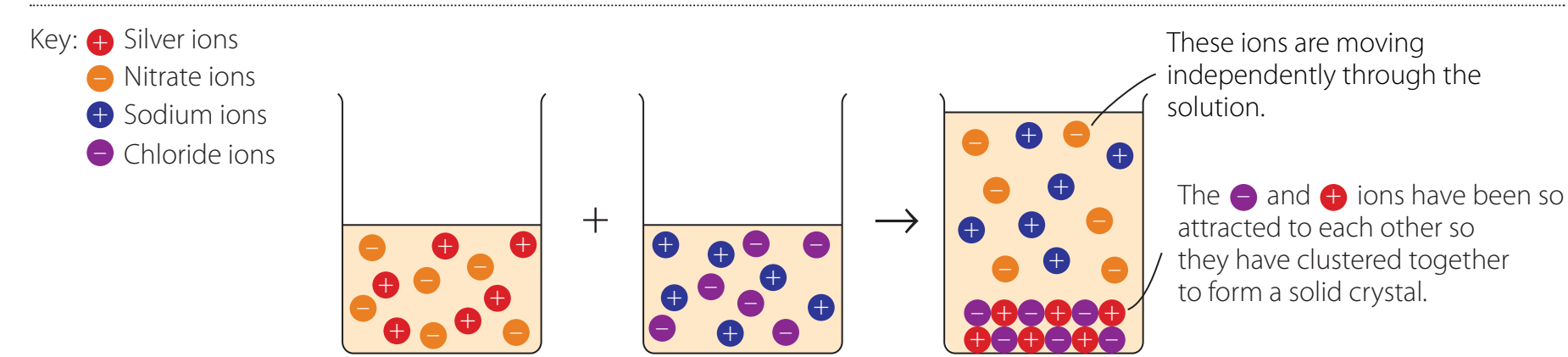
Net Ionic Equations
- Net Ionic Equations: Focus on the ions actively involved in the precipitation process, excluding spectator ions for clarity.
Steps for Writing Net Ionic Equations
- Step 1: Begin with a balanced molecular equation.
- Step 2: Disassociate soluble compounds into their ions to form the complete ionic equation.
- Step 3: Identify and eliminate spectator ions.
- Step 4: Draft the net ionic equation, emphasising the ions resulting in the precipitate.

Example: Reaction between AgNO₃ and NaCl
- Molecular Equation:
- Complete Ionic Equation:
- Net Ionic Equation:
Laboratory Investigation
Safety Precautions
- Gloves: Protect hands from corrosive substances.
- Goggles: Shield eyes from chemical splashes.
- Lab Coats: Safeguard clothing and skin from spills.
Lab Safety Rules: Always wear appropriate protective gear to minimise risks.
Essential Laboratory Equipment
- Pipettes: Used for transferring accurate liquid volumes.
- Burettes: Utilised in titrations for precise measurements.
- Beakers: Serve for general-purpose mixing.
- Test Tubes: Suitable for conducting small-scale reactions.

Procedure for Precipitation Reactions
- Select chemicals according to solubility rules.
- Measure quantities with precision.
- Mix solutions and examine reactions.

Equilibrium and Ksp
- Ksp (Solubility Product Constant): Indicates a compound's solubility, aiding in the prediction of precipitation.
- Example: Ksp of AgCl is , indicating when silver chloride will precipitate.
Ion Product (Qsp)
- Comparison: Assesses the potential for precipitation.
- Qsp = Ksp: Equilibrium condition.
- Qsp < Ksp: Unsaturated solution, no precipitation.
- Qsp > Ksp: Supersaturated solution, resulting in precipitation.
Example: Determining Precipitation in Solution Mixing
Scenario: Combining 100 mL of 0.02 M AgNO₃ with 100 mL of 0.02 M KCl.
Solution:
-
Calculate final concentrations:
- Volume doubles to 200 mL, so both AgNO₃ and KCl are diluted by half
- [Ag⁺] = 0.01 M
- [Cl⁻] = 0.01 M
-
Calculate Qsp:
- Qsp = [Ag⁺][Cl⁻] = 0.01 × 0.01 = 1 × 10⁻⁴
-
Compare with Ksp of AgCl (1.8 × 10⁻¹⁰):
- Since Qsp > Ksp (1 × 10⁻⁴ > 1.8 × 10⁻¹⁰), precipitation will occur
Example: Na₂SO₄ and BaCl₂ Reaction
Solution:
-
Write the molecular equation:
- Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
-
Determine if precipitation occurs:
- From solubility rules, BaSO₄ is insoluble
- Precipitation will occur when solutions are mixed
-
Write the net ionic equation:
- Ba²⁺(aq) + SO₄²⁻(aq) → BaSO₄(s)
Common Mistakes and Troubleshooting
Errors in Precipitation Reactions
- Incorrect determination of spectator ions.
- Neglecting to balance equations.
Ensure accurate identification and elimination of spectator ions in net ionic equations.
Practice Problems with Solutions
-
Predict Precipitation upon mixing Na₂SO₄ with BaCl₂.
- Solution: Yes, precipitation will occur. BaSO₄ forms as an insoluble white precipitate because barium sulfate is insoluble according to solubility rules.
-
Construct the Net Ionic Equation for CaCl₂ with K₂CO₃ resulting in CaCO₃.
- Solution:
- Molecular equation: CaCl₂(aq) + K₂CO₃(aq) → CaCO₃(s) + 2KCl(aq)
- Complete ionic equation: Ca²⁺(aq) + 2Cl⁻(aq) + 2K⁺(aq) + CO₃²⁻(aq) → CaCO₃(s) + 2K⁺(aq) + 2Cl⁻(aq)
- Net ionic equation: Ca²⁺(aq) + CO₃²⁻(aq) → CaCO₃(s)
- Solution:
500K+ Students Use These Powerful Tools to Master Precipitation Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
209 flashcards
Flashcards on Precipitation Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Precipitation Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes23 questions
Exam questions on Precipitation Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Precipitation Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Precipitation Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Precipitation Reactions you should explore
Discover More Revision Notes Related to Precipitation Reactions to Deepen Your Understanding and Improve Your Mastery
