Photo AI
Last Updated Sep 24, 2025
Combustion Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Combustion Reactions quickly and effectively.
290+ students studying
Combustion Reactions
Combustion reactions are essential chemical processes in both industrial and natural settings. These reactions occur when substances react with oxygen, releasing energy as heat and light, often resulting in high temperatures and visible flames.
Definition and General Characteristics
Combustion Reaction: A chemical process where substances react with oxygen, releasing energy as heat and light, frequently resulting in elevated temperatures and visible flames.
- General Characteristics:
- Involves reactions with oxygen.
- Produces heat and light.
- Occurs rapidly, often resulting in visible flames.
Typical Reactants
- Hydrocarbons and Metals:
- Common reactants include hydrocarbons such as methane and metals like magnesium.
- Examples with Equations:
- Methane Combustion:
- Methane reacts with oxygen.
- Produces carbon dioxide and water.
- Releases heat and light.
- Magnesium Combustion:
- Magnesium reacts with oxygen.
- Forms magnesium oxide.
- Emission of light and heat.
- Methane Combustion:
Energy Changes
- Exothermic Nature: Combustion reactions are exothermic. Their main outputs include:
- Heat.
- Light.
Combustion Products
- Primary Products:
- Carbon dioxide (CO₂) and water (H₂O) are typically produced.
- Types of combustion:
- Complete Combustion: Achieves full conversion to CO₂ and H₂O.
- Incomplete Combustion: May lead to the formation of carbon monoxide (CO) and soot.
Visual Aids and Interpretations
-
Visual Interpretations:
- Observe how flame colour and reaction intensity indicate the efficiency of combustion.
-
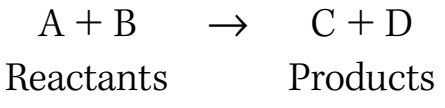
- Explanation: Highlights the stages in a combustion reaction, depicting the interaction of reactants to form products.
-

- Explanation: Visualise reaction processes and associated energy releases, noting the visual markers of combustion intensity.
Complete Combustion
Complete Combustion: Occurs with an adequate supply of oxygen, enabling hydrocarbons to react and generate carbon dioxide (CO₂) and water (H₂O).
Chemical Equations
- Propane (C₃H₈) Example
- Formula:
- Propane combusts to produce carbon dioxide and water.
- Formula:
- Octane (C₈H₁₈) Example
- Formula:
- Octane combusts to yield carbon dioxide and water.
- Formula:
Characteristics
- Energy: Produces maximum energy per mole of reactants.
- Flame Colour: Blue flame indicates efficient combustion.
Incomplete Combustion
Incomplete Combustion: Occurs with insufficient oxygen, forming carbon monoxide (CO), soot, and water instead of only CO₂ and H₂O.
Chemical Equations
- Methane (CH₄) Example
- Formula:
- Methane combusts producing carbon monoxide and soot.
- Formula:
Characteristics
- Flame Colour: Yellow or orange, due to glowing carbon particles.
- Energy: Produces less energy compared to complete combustion.
Environmental and Health Implications
Complete Combustion
- Impact: Produces CO₂, contributing to climate change by intensifying the greenhouse effect.
Incomplete Combustion
- Toxicity: Produces CO, posing severe health risks by potentially causing respiratory issues.
- Pollution: Soot contributes to air pollution and can harm respiratory health.
- Energy Output: Higher in complete combustion compared to incomplete combustion.
- Flame Colour: Blue for complete combustion; yellow/orange for incomplete combustion.
- Safety: Incomplete combustion poses significant health risks due to carbon monoxide production.
Practical Investigations in Combustion
Understanding combustion reactions is crucial in both academic contexts and practical applications such as energy production and pollution management. This investigation bridges classroom knowledge with real-world benefits, like cleaner technologies.
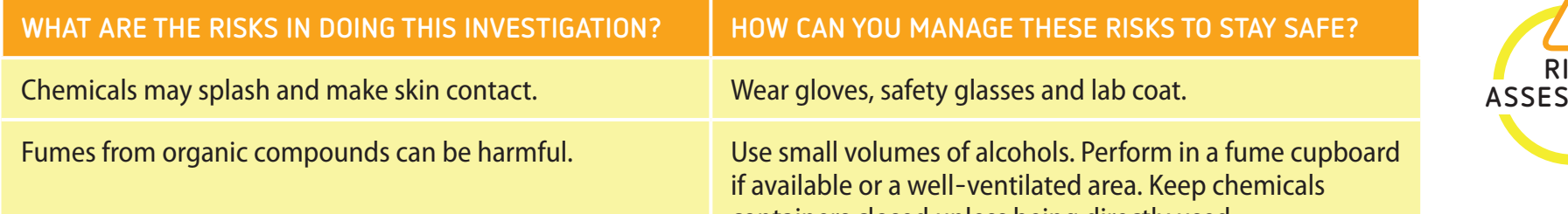
Equipment and Safety Gear
- Essential Equipment:
- Bunsen burner: Provides a consistent flame, essential for reliable results.
- Test tubes: Used to safely contain and observe chemical reactions.
- Fume hood: Protects by ventilating toxic fumes, crucial for safe handling of reactive substances.
- Safety Gear:
- Safety goggles: Prevent eye damage from splashes or fumes.
- Lab coat and gloves: Shield against chemical spills and direct contact.
Safety Scenario Reminder: Avoid mishaps, such as burns from acid splashes, by wearing proper safety gear—similar to a cyclist wearing a helmet for head protection.
Experimental Setup and Safety
- Ensure the Bunsen burner is securely placed under the fume hood, with a properly adjusted flame to prevent safety hazards.
- Arrange test tubes carefully in the holder under the hood ensuring no spills.
- Double-check all safety equipment before proceeding.
Troubleshooting:
- If the burner flame is yellow, adjust the gas-air mixture to obtain a blue flame for optimal combustion.
Experimentation Procedure
- Procedure Steps:
- Ignite the Bunsen burner carefully. Control the flame size, as you would adjust a cooker, for desired reaction conditions.
- Introduce reactants carefully while observing changes such as colour or gas emissions.
- Use tools to test for gaseous products or residue.
- Common error: Misaligned test tubes can disrupt controlled reactions; ensure the setups are correct.
Guidelines for Accurate Product Identification
- Indicators include: colour changes, smoke formation, residue detection.
- Reactants and Products Table:
| Reactant Type | Expected Products |
|---|---|
| Hydrocarbon | CO₂ and H₂O |
| Metals | Metal oxides |
- Practical Example: The glow from burning wood links observed soot to lab-identifiable soot residue.

Conclusion of Practical Investigation
Understanding combustion products is vital for applying scientific knowledge to societal benefits, such as the development of effective heating systems.
Real-World Application: Modern heating solutions utilise combustion insights to improve efficiency and safety, reducing home energy costs.
Summary
Exam Tips
- Understand the differences between complete and incomplete combustion reactions.
- Recognise visual and chemical indicators of combustion efficiency.
- Pay attention to safety protocols and proper lab techniques.
500K+ Students Use These Powerful Tools to Master Combustion Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
209 flashcards
Flashcards on Combustion Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Combustion Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes23 questions
Exam questions on Combustion Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Combustion Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Combustion Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Combustion Reactions you should explore
Discover More Revision Notes Related to Combustion Reactions to Deepen Your Understanding and Improve Your Mastery
