Photo AI
Last Updated Sep 24, 2025
Decomposition Reactions Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Decomposition Reactions quickly and effectively.
238+ students studying
Decomposition Reactions
Overview
- Decomposition Reactions: A process in which a compound separates into two or more simpler substances.
- Significance: Essential for predicting the products of reactions in chemistry.
Syllabus Connection: Understanding decomposition reactions is crucial for solving examination problems and performing well in laboratory work.
Characteristics and Types of Decomposition Reactions
Overview
- Decomposition reactions entail the breakdown of a compound into simpler substances with the input of energy, such as heat, light, or electricity, which is necessary to break the chemical bonds in the compounds.
Types of Decomposition Reactions
Thermal Decomposition
- Description: Involves the breakdown of compounds through the application of heat, initiated by high temperatures.
- Example: Calcium carbonate () decomposes into calcium oxide () and carbon dioxide ().
- Chemical Equation:
- Key Factors: Temperature provides the necessary energy to disrupt chemical bonds.
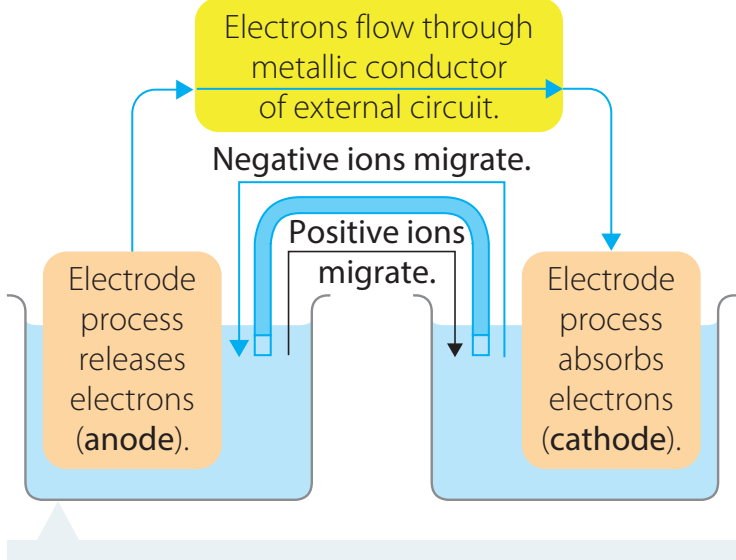
Electrolytic Decomposition
- Description: Utilises electricity to break down compounds.
- Example: Electrolysis of water yields hydrogen and oxygen gases.
- Chemical Equation:
- Key Factors: Electric current intensity affects the rate and success of the decomposition.
Photolytic Decomposition
- Description: Decomposition initiated by light, where photons provide the energy required for bond breakage.
- Example: Silver chloride () decomposes under exposure to sunlight, yielding silver and chlorine gas.
- Chemical Equation:
- Key Factors: Light wavelength and intensity are critical for effective bond cleavage.

Comparative Analysis of Decomposition Types
| Type of Reaction | Required Conditions | Common Examples | Energy Sources |
|---|---|---|---|
| Thermal | - Elevated temperature | - CaCO → CaO + CO | - Heat |
| Electrolytic | - Electric current and electrolyte presence | - Water → H + O | - Electricity |
| Photolytic | - Suitable light wavelength and intensity | - AgCl under sunlight → Ag + Cl | - Light |
Practice Question Highlights
Sample Questions:
- Question 1: Determine the type of decomposition:
- Solution: Thermal Decomposition - This reaction requires heat to break down mercury(II) oxide into its constituent elements.
- Question 2: Justify the necessity of an electric current for water to decompose into hydrogen and oxygen.
- Solution: Electric current enables ion migration through the water, providing the energy needed to overcome the strong bonds in water molecules, which is necessary for decomposition into hydrogen and oxygen.
Exam Tips:
- Pitfalls to Avoid: Confusing reactions with similar initiators.
- Common Misconceptions: Appreciating the subtle roles of energy sources in enabling decomposition.
Balancing Chemical Equations
- Importance: Balancing ensures that the Law of Conservation of Mass is honoured.
Balancing ensures mass conservation across reactions.
Example of Balancing
- Start with the unbalanced equation:
- Count the number of atoms for each element on both sides:
- Left side: 1 K, 1 Cl, 3 O
- Right side: 1 K, 1 Cl, 2 O (in O₂)
- Adjust coefficients to achieve balance:
- We need to balance oxygen atoms first
- Since O₂ has 2 oxygen atoms, and we need 3 oxygen atoms, we need to find numbers that work
- If we have 2 KClO₃ molecules (with 6 O atoms) and 3 O₂ molecules (with 6 O atoms), we achieve balance
- This gives us: 2 K, 2 Cl on the left and 1 K, 1 Cl on the right
- So adjust KCl to 2KCl to balance K and Cl atoms
- Final balanced equation:

Insights and Misconceptions
Common Misconceptions in Decomposition Reactions
1. Differentiating Between Decomposition Types
-
Thermal Decomposition:
- Initiator: Heat.
- Example: .
-
Electrolytic Decomposition:
- Initiator: Electricity.
- Example: .
-
Photolytic Decomposition:
- Initiator: Light.
- Example: .
2. Reaction Conditions
- Grasping reaction conditions is crucial:
- Self-check Questions:
- "Which condition drives this reaction?"
- "How does temperature affect the reaction process?"
- Self-check Questions:
3. Balancing Equations
- Steps to Minimise Errors:
- Enumerate all reactants and products.
- Count the number of atoms for each element.
- Modify coefficients for balance.
Balancing Tip:
- Focus on the most complex molecule first.
- Balance one element's atoms at a time.
- Verify your work thoroughly.

Experimental Procedures for Thermal Decomposition
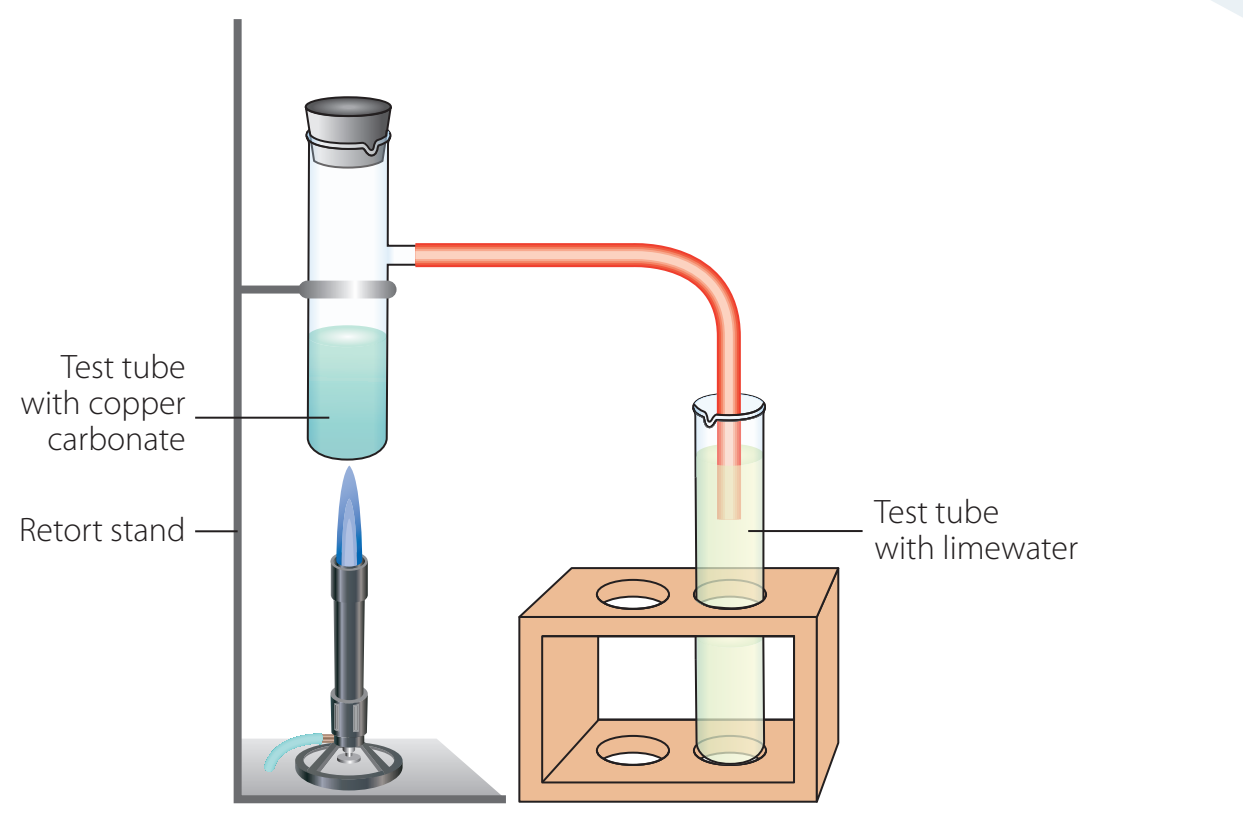
Gathering Materials:
- Collect copper carbonate, a heat source such as a Bunsen burner, and a gas collection apparatus.
- Confirm that essential personal protective equipment (PPE) such as gloves, goggles, and a lab coat, is accessible.
Setting Up Apparatus:
- Safely configure the apparatus for secure heating and gas capture.
- Ensure all connections are firm and PPE is properly donned.
Safety Protocols and Best Practices
PPE is compulsory: gloves, goggles, and lab coat must be worn at all times.
Safety Importance and Error Prevention
- Incorrect Balancing of Equations: Can lead to setup errors; always verify equations.
- Incomplete Gas Collection System: Ensure all connections are dependable to inhibit gas leakage.
Feedback has shown that not securing the apparatus properly is a common student mistake. Double-check setups to avoid this error.
Following these guidelines and setups allows students to safely and effectively conduct experimental studies of thermal decomposition.
500K+ Students Use These Powerful Tools to Master Decomposition Reactions For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
209 flashcards
Flashcards on Decomposition Reactions
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards27 quizzes
Quizzes on Decomposition Reactions
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes23 questions
Exam questions on Decomposition Reactions
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Decomposition Reactions
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Decomposition Reactions
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Decomposition Reactions you should explore
Discover More Revision Notes Related to Decomposition Reactions to Deepen Your Understanding and Improve Your Mastery
