Photo AI
Last Updated Sep 27, 2025
¹H NMR Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand ¹H NMR quickly and effectively.
353+ students studying
7.10.2 ¹H NMR
High-Resolution ¹H NMR (Proton NMR) Spectroscopy
NMR (Proton NMR) is a technique used to analyze the environments of hydrogen () atoms in a molecule, providing detailed information on molecular structure. The data gathered from a proton NMR spectrum, including chemical shifts, integration, and splitting patterns, can help identify the structure of unknown compounds.
Sample Preparation for ¹H NMR
- Solvents: ¹H NMR spectra are obtained using samples dissolved in deuterated solvents (such as CDCl₃ or D₂O) or carbon tetrachloride (CCl₄). Deuterated solvents are used because deuterium (²H) does not produce signals in the same range as ¹H, preventing interference.
- Comparison with : Unlike , which provides simpler spectra (usually without splitting due to carbon's low abundance and single-bonded nature), NMR spectra are more complex due to interactions between neighbouring hydrogen atoms.
Key Principles in NMR Analysis
- Chemical Shifts:
- The chemical shift of each peak indicates the type of hydrogen environment. Chemical shifts are measured in parts per million (ppm) and are influenced by the electronic environment around the hydrogen atoms.
- Shielded protons (those surrounded by electron density) appear at lower ppm (upfield), while deshielded protons (close to electronegative atoms or π-bonds) appear at higher ppm (downfield).
- Use the Chemistry Data Booklet to interpret chemical shift values and suggest possible structures or parts of molecules.
- Integration:
- The integration of each peak reflects the relative number of equivalent protons in that environment. Integration ratios indicate the simplest whole-number ratio of hydrogen atoms present in each unique environment.
- For example, a peak with an integration value of 3 represents three equivalent hydrogens, such as those in a group.
- Spin-Spin Coupling and the Rule:
- Spin-spin coupling occurs when hydrogen atoms on adjacent carbon atoms influence each other's magnetic environment, causing the splitting of peaks.
- Rule: The number of peaks in each signal is determined by the number of neighbouring, non-equivalent protons (n), resulting in a splitting pattern of .
- Singlet: No neighboring H atoms (e.g., isolated CH₃), appear as a single peak.
- Doublet: One neighbouring H atom, resulting in two peaks.
- Triplet: Two neighbouring H atoms, resulting in three peaks.
- Quartet: Three neighbouring H atoms, resulting in four peaks.
Spin-spin coupling patterns provide information about the number of adjacent protons, helping deduce the molecular structure.
Steps to Interpret a High-Resolution ¹H NMR Spectrum
- Count the Peaks:
- The total number of peaks (or sets of peaks if split) represents the number of distinct hydrogen environments within the molecule.
- Use Integration Ratios:
- If integration data is provided, use the integration ratio to determine the relative number of protons in each environment. This ratio often helps identify functional groups, such as , , or groups.
- Interpret Singlets First:
- Start by examining any singlet peaks, as they represent hydrogen environments with no adjacent protons:
- For example, a singlet with an integration of 1 and a chemical shift of 1–5 ppm could indicate an group.
- A singlet with an integration of 3 typically indicates a CH₃ group with no neighbouring hydrogens, producing a single peak.
- Consider Other Peaks Using Splitting Patterns and Chemical Shifts:
- Analyze each peak's splitting pattern to determine the number of neighboring protons (using the ( n+1 ) rule). Use the chemical shift values to suggest possible functional groups for each environment.
- For example, a triplet around 0.9 ppm might indicate an R–CH₃ group adjacent to a CH₂ group.
- Determine the Number of Adjacent Hydrogens:
- Use the splitting pattern of each peak to deduce the number of neighboring hydrogens on adjacent carbon atoms. For instance, a quartet indicates a group adjacent to a group.
- Construct the Structure:
- Combine all information from the chemical shifts, integration ratios, and splitting patterns to deduce the molecular structure.
Worked Example: Deduction of Molecular Structure from ¹H NMR Data
Suppose the ¹H NMR spectrum is given for an alcohol with the molecular formula . Here's how you might deduce its structure:
- Identify Peaks: The spectrum shows four distinct peaks, indicating four unique hydrogen environments.
- Integration Ratios: The integration values are 1.6 : 0.4 : 1.2 : 2.4, which can be simplified to 4 : 1 : 3 : 6.
- Analyze Singlets:
- A singlet with an integration of 1 and a chemical shift around 1.1 ppm likely corresponds to the OH group.
- Interpret Remaining Peaks:
- A singlet with an integration of 3 and a chemical shift around 1.2 ppm is likely a CH₃ group with no adjacent protons.
- A triplet near 0.9 ppm suggests an group next to a group, as triplets often indicate two neighboring hydrogens.
- Construct the Structure:
- Using this data and possible configurations, you would deduce the structure of the molecule to satisfy the peak pattern, integration, and molecular formula.
Hydrogen Environments
- Each peak on a 1H NMR spectrum is due to one or more protons in a particular environment the relative area under each peak tells you the relative number of H atoms in each environment.
Number of Signals - Number of Different Hydrogen Environments
H atoms that appear at the same δ on the spectra are in the same environment within the molecule said to be chemically equivalent. • Chemically equivalent H atoms are not distinguishable on an NMR spectrum.
Relative Intensity of Signals - Number of Equivalent Hydrogen Atoms
• In 1H NMR (but not 13C NMR) the area of the signal is proportional to the number of H atoms it represents. • There are many ways in which the relative size (area) of the signals can be shown • The most common one is to indicate the relative intensity of the signals • We can use this to find the simplest whole number ratio can be calculated. - E.g. relative intensity = 0.3 : 0.15 : 0.3 : 0.6 = 2 : 1 : 2 : 6
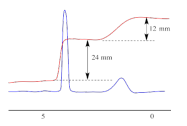
Splitting Pattern
-
Equivalent 1H nuclei will appear at the same chemical shift value on the spectrum. However the peak for these 1H nuclei will be split into a number of peaks depending on the number of non-equivalent 1H nuclei bonded to the adjacent C atoms.
-
These split peaks are called multiples. Note: the H atom of the OH group in alcohols rarely causes splitting, or is split itself.
-
Sometimes the H atom of an OH group appears as a broad hump.
-
Splitting of the peaks into a set of peaks is known as the splitting pattern.
-
The splitting pattern follows the n+1 rule This says that if, for example, there's 4 1H nuclei on adjacent C atoms you get 5 peaks
If...
• 0 non-equivalent protons are bonded to the adjacent C atoms, peak is not split = singlet. • 1 non-equivalent proton is bonded to adjacent C atoms, peak is split into 2 smaller peaks (smaller as no. of peaks in env. unchanged therefore integration must be the same) = doublet. • 2 non-equivalent protons are bonded to adjacent C atoms, peak is split into 3 smaller peaks = triplet. • If 3 non-equivalent protons are bonded to adjacent C atoms, peak is split into 4 smaller peaks = quartet.
Proton-free Solvents
- The solvent used to dissolve compounds for 1H NMR spectroscopy is tetrachloromethane ()
- does not contain 1H nuclei so doesn't give peaks on the spectrum.
Predicting Structure from 1H NMR spectra
Here is a summary to help you analyse the structure from 1H NMR spectra:
Number of peaks
- Tells you the number of different H environments.
• Ratio of peak areas
- Tells you the relative number of H atoms in each environment.
• Chemical shifts
- Tells you the type of environment the H is in.
• Splitting pattern
- Tells you the number of H atoms on the adjacent C.
E.g. Using the spectrum below, + the table of chemical shift data, predict the structure of the compound.
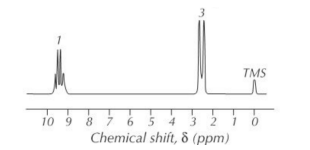
- Number of peaks
- 2 peaks - A and B
• Ratio of peak areas
- A : B = 1 : 3
• Chemical shifts
- δ of A ≈ 9.5 ppm ∴
- δ of B ≈ 2.5 ppm ∴
• Splitting pattern
- A = quartet so this proton has 3 neighbouring protons. - B = doublet so these protons have 1 neighbouring proton. - therefore A + B are next to each other. So this is the 1H NMR spectrum for ethanal ().
500K+ Students Use These Powerful Tools to Master ¹H NMR For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on ¹H NMR
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards29 questions
Exam questions on ¹H NMR
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on ¹H NMR
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on ¹H NMR
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to ¹H NMR you should explore
Discover More Revision Notes Related to ¹H NMR to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Nuclear Magnetic Resonance Spectroscopy (A Level only)
Principles of NMR
291+ studying
194KViews96%
114 rated
Nuclear Magnetic Resonance Spectroscopy (A Level only)
¹³C NMR
220+ studying
188KViews