Photo AI
Last Updated Sep 27, 2025
Principles of NMR Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand Principles of NMR quickly and effectively.
247+ students studying
7.10.1 Principles of NMR
Principles of Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the molecular structure of compounds by examining the environments of specific atomic nuclei. NMR provides information about the positions of certain isotopes, such as and , within a molecule, which helps chemists confirm the structure of new compounds.
Basic Principle of NMR
- NMR Mechanism: NMR operates by applying a strong magnetic field to nuclei that behave as tiny magnets due to their charge and intrinsic spin. Atoms with nuclei possessing an odd number of protons or neutrons (such as and exhibit magnetic properties, which are key to NMR analysis.
- Spin States: When a nucleus is exposed to an external magnetic field, it can align either with the field (low-energy α-spin state) or against it (high-energy β-spin state).
- Resonance: When the nuclei absorb energy from applied radiofrequency radiation, they transition from the α-spin state to the β-spin state. This process of absorbing energy to flip spin states is called resonance, which is the basis of NMR.
Shielded and Deshielded Nuclei
- Electronic Environments: Nuclei in different electronic environments experience varying degrees of shielding. Nuclei surrounded by electron clouds are shielded from the external magnetic field, while those with fewer electrons (due to electronegative atoms nearby) are deshielded.
- Effect on Resonance:
- Shielded nuclei require less energy to achieve resonance because the electron cloud around them reduces the impact of the magnetic field.
- Deshielded nuclei need more energy for resonance as they experience a stronger effective magnetic field.
- Energy Requirement: Nuclei in different environments (shielded vs. deshielded) absorb different amounts of energy, which is detected and represented in the NMR spectrum.
Interpreting the NMR Spectrum
- Peaks and Signals: Each peak or signal in an NMR spectrum represents the energy required to bring a particular nucleus into resonance.
- Upfield (Shielded): Peaks for shielded nuclei appear upfield (lower energy).
- Downfield (Deshielded): Peaks for deshielded nuclei appear downfield (higher energy).
Example: In , the presence of an electronegative chlorine atom deshields the nearby CH₂ protons, while the protons are further away and more shielded. This difference creates two distinct peaks on the NMR spectrum.
Key Equation for Energy (ΔE)
The energy required to bring a nucleus from the low-energy state (α-spin) to the high-energy state (β-spin) can be calculated with the formula:
where:
- is Planck's constant,
- is the frequency of the applied radiofrequency radiation.
Applications of NMR
- Structural Determination: NMR is widely used to deduce the structure of organic compounds, helping to identify functional groups and molecular arrangements. For example:
- 1-Bromopropane: NMR can distinguish between 1-bromopropane and 2-bromopropane by examining the shielding effects caused by bromine.
- In 1-bromopropane, there are three distinct environments for hydrogen atoms, resulting in three peaks.
- In 2-bromopropane, only two environments exist, resulting in two peaks.
- Medical Imaging: NMR forms the basis of magnetic resonance imaging (MRI), a non-invasive technique that provides detailed images of internal body structures, including soft tissues. MRI is valuable in medicine as it does not use harmful radiation, unlike CT scans.
Why do we use 13C and 1 H; How does NMR work?
Why?
13C or 1H atoms are used because they are atoms with nuclear spin. • This is a quantum property, much more undergrad level than a-level • Essentially though, 13C and 1H behave like magnets when they themselves are placed in a strong magnetic field. • So, when the nuclei are placed in a magnetic field, the magnetic (spin) line up parallel to the applied field • This happens in one of two ways: • Spin aligned (lined up with the external field) = lower energy. • Spin opposed (lined up against the external field) = higher energy.
How? • In an NMR spectrometer, EM radiation of a specific frequency (depending on the nuclei in question) is absorbed by the nuclei • It's used to 'flip' the alignment of nuclear spin from the lower energy spin to the higher energy spin state. • The energy re-emitted from the nuclei (as the alignment of their nuclear spins drop from a higher to a lower energy state) is absorbed by the spectrometer. • This EXACT energy can be measured and processed, producing an NMR spectrum.
Chemical Shift
- The x-axis of an NMR spectrum is chemical shift (δ)
- It's measured in ppm (parts per million) and the scale increases from right to left.
- The chemical shift is the difference in the energy absorbed by nuclei in different environments relative to a standard (with δ of 0)
The tetramethylsilane molecule:
-
The standard substance is TMS (tetramethylsilane) for both 1H and a 13C NMR spectrum TMS is a suitable substance to use as a standard because:
-
It Gives a signal that is further right (closer to 0) than most of the signals from organic compounds (this is thanks to the electronegativity of Si). • It Only gives one signal as all 12 H atoms are chemically equivalent and all 4 C atoms are also chemically equivalent. • It Is non-toxic and inert. • It Has a low boiling point (it's volatile) so can be easily removed from the sample afterwards.
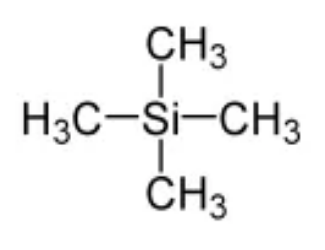
tetramethylsilane
- An NMR spectrum allows the structure of molecules to be determined because nuclei positioned in different environments will give rise to separate signals • These separate signals will lead to separate peaks with different . • The strength of the external magnetic field is not the only variable that affects • it also depends on molecular environment
- Proximity of nuclei to areas of high electron density or other electronegative nuclei - Increased electron density or number of electronegative nuclei
500K+ Students Use These Powerful Tools to Master Principles of NMR For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on Principles of NMR
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards3 quizzes
Quizzes on Principles of NMR
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Principles of NMR
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Principles of NMR
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on Principles of NMR
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Principles of NMR you should explore
Discover More Revision Notes Related to Principles of NMR to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Nuclear Magnetic Resonance Spectroscopy (A Level only)
¹H NMR
380+ studying
193KViews96%
114 rated
Nuclear Magnetic Resonance Spectroscopy (A Level only)
¹³C NMR
475+ studying
191KViews