Photo AI
Last Updated Sep 27, 2025
¹³C NMR Simplified Revision Notes for A-Level AQA Chemistry
Revision notes with simplified explanations to understand ¹³C NMR quickly and effectively.
421+ students studying
7.10.3 ¹³C NMR
¹³C NMR Spectroscopy (Carbon-13 NMR)
¹³C NMR spectroscopy is a technique used to analyse the environments of carbon atoms in a molecule, providing information about the molecular structure. It is generally simpler to interpret than ¹H NMR due to the low natural abundance of ¹³C nuclei and the fact that ¹³C nuclei are unlikely to be bonded to each other, reducing complex coupling patterns.
- A spectrum is simply a number of peaks, each peak corresponds to an environment of equivalent 13C nuclei.
- Environments are determined by the structural symmetry of the molecule
- So, this determines the number of peaks. Similar to 1H NMR, electronegative atoms attached to C atoms in molecules leads to those C atoms having higher δ values.
Key Principles of ¹³C NMR
- Carbon Environments and Peaks:
- Each peak on a ¹³C NMR spectrum represents a unique carbon environment in the molecule.
- The number of peaks corresponds to the number of distinct carbon environments.
- Example: In ethanol (), there are two different carbon environments: one for the CH₃ group and another for the group, resulting in two peaks on the ¹³C NMR spectrum.
- Determining the Number of Carbon Environments:
- To interpret a ¹³C NMR spectrum, the first step is to count the number of peaks. This tells us how many different types of carbon environments are present in the molecule.
- Carbons in identical chemical environments produce a single peak, even if there are multiple equivalent carbons.
Examples of Interpreting ¹³C NMR Spectra
How many peaks on the ¹³C NMR would you expect on the molecules below?
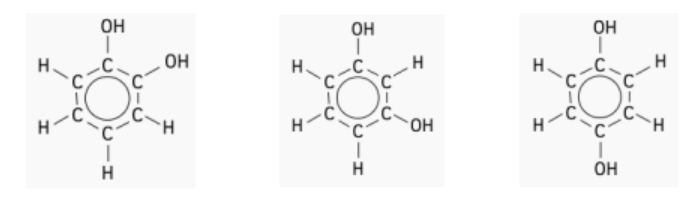
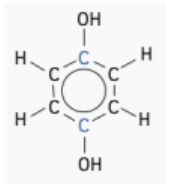
- Molecule A:
- There are three different types of carbon environments.
- The carbon atoms attached to the group are in identical environments (colored blue).
- The carbon atoms next to the carbons are in a different, but identical, environment to each other (coloured black).
- Remaining carbons are in a third, identical environment (colored red).
- Expected Peaks: 3 peaks, one for each type of carbon environment.
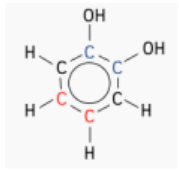
- Molecule B:
- Four distinct carbon environments exist.
- Two carbons are in the same environment (colored blue), while two others are in a different shared environment (colored black).
- One carbon is unique (colored green) as it is adjacent to two bonds.
- Expected Peaks: 4 peaks for the four distinct environments.
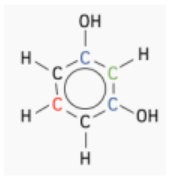
- Molecule C:
- There are two unique carbon environments.
- Two carbons share an environment (colored blue).
- The remaining four carbons are in another identical environment, each adjacent to a group.
- Expected Peaks: 2 peaks, corresponding to the two unique carbon environments.
Using Chemical Shifts in ¹³C NMR
Carbon NMR is a valuable tool for identifying functional groups based on characteristic chemical shift values. The ¹³C chemical shifts are significantly influenced by the presence of electronegative atoms. When a hydrogen atom in an alkane is replaced by a substituent, such as an electronegative atom (e.g., O, N, or a halogen), the ¹³C signals of nearby carbons shift downfield (to the left, indicating an increase in ppm). This downfield shift effect decreases with increasing distance from the electron-withdrawing group. Figure 13.11.1 illustrates the typical ¹³C chemical shift ranges for major chemical classes.
- The chemical shift in a ¹³C NMR spectrum depends on the electronic environment around each carbon.
- Electronegative Groups: The more electronegative the group attached to a carbon, the higher the chemical shift (further downfield from the TMS reference at 0 ppm).
- Chemical Shift Data: By comparing the chemical shift values of peaks with standard data (e.g., from the AQA Chemistry Data Booklet), you can identify the types of carbon environments.
Spin-Spin Splitting in ¹³C NMR
There is a key difference between ¹H NMR and ¹³C NMR in terms of spin-spin splitting. ¹³C-¹³C spin-spin splitting between adjacent carbons is extremely rare because ¹³C is naturally low in abundance (about 1.1%).
¹³C-¹H Spin Coupling
¹³C-¹H spin coupling provides valuable information about the number of protons attached to each carbon atom. In cases of one-bond coupling ():
- groups appear as doublets,
- groups appear as triplets,
- groups appear as quartets. However, ¹³C-¹H spin coupling has a drawback: it complicates the ¹³C NMR spectrum due to a large number of overlapping peaks. This "forest" of peaks results from the high natural abundance of ¹H (100%), making the spectrum difficult to interpret.
Decoupling
Decoupling is a technique used to simplify the ¹³C NMR spectrum by removing the ¹³C-¹H coupling interactions. This allows each unique carbon to appear as a single peak (singlet) in the spectrum, making it easier to identify distinct carbon environments. In decoupled ¹³C spectra, each carbon shows as a singlet, regardless of the number of attached protons.
Decoupling is achieved by irradiating at the frequency of a specific proton using continuous low-power radiofrequency (RF) waves, effectively removing the coupling effect. This method provides a clearer spectrum that highlights the individual carbon environments.
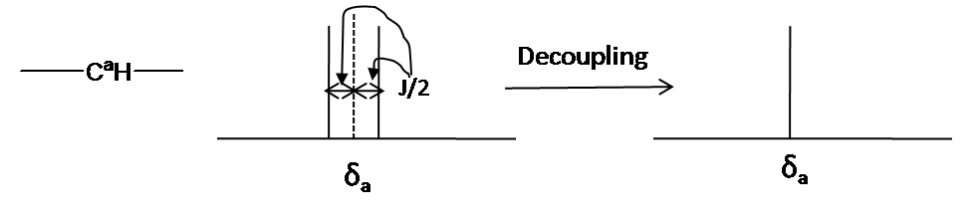
*Decoupling in the 13C NMR *
Steps for Analyzing a ¹³C NMR Spectrum
- Count the Peaks:
- Identify the number of peaks to determine the number of unique carbon environments.
- Match Peaks with Chemical Shift Values:
- Compare the chemical shifts of the peaks to known values to suggest the possible groups each carbon is bonded to.
- Interpret Using Additional Information:
- Sometimes chemical shifts overlap, so it may be necessary to use other details (like molecular formula or structural hints) to confirm the identity of each carbon environment.
Worked Example: Consider a molecule with a cyclic structure and molecular formula. The ¹³C NMR spectrum shows four distinct peaks.
- Interpretation:
- Four Peaks indicate four different carbon environments.
- Cyclic Structure suggests symmetry, which may result in equivalent carbons within the ring.
- The chemical shifts can help identify whether carbons are attached to oxygen or involved in different bonding patterns.
Predicting Structure from 13C NMR spectra
Number of peaks
-
Tells you the number of different C environments Chemical shifts
-
Tells you what kind of C environment is causing each peak
-
E.g. The 13C NMR spectrum of a straight-chain molecule with the molecular formula C5H10O is shown below.
-
Using the spectrum, and the table of chemical shift data, identify the molecule.
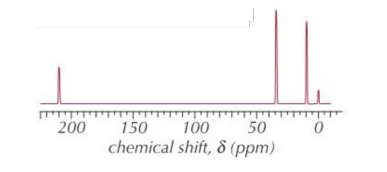
- No. of peaks
- 3 peaks - A, B and C
• Chemical shifts
- δ of A ≈ 210 ppm so R-CO- (ketone or aldehyde) - δ of B ≈ 35 ppm so R-CO-C- - δ of C ≈ 10 ppm so C-C So this is the 13C NMR spectrum for pentan-3-one ().
13C NMR spectra of Cyclic Molecules
The number of peaks on the 13C NMR spectrum of a cyclic compound depends on the symmetry of the molecule.
E.g. The 13C NMR spectrum of a cyclic molecule with the formula C6H4Cl2 is shown below. Identify the molecule that produced this spectrum.
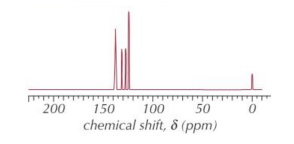
- No. of peaks
- 4 peaks
• Chemical shifts
- δ of 4 peaks are between 125 ppm and 140 ppm SO there's a benzene ring. There are only 3 aromatic molecules with the formula
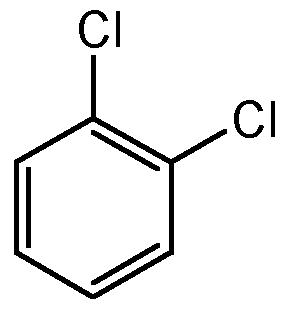
1,2-Dichlorobenzene
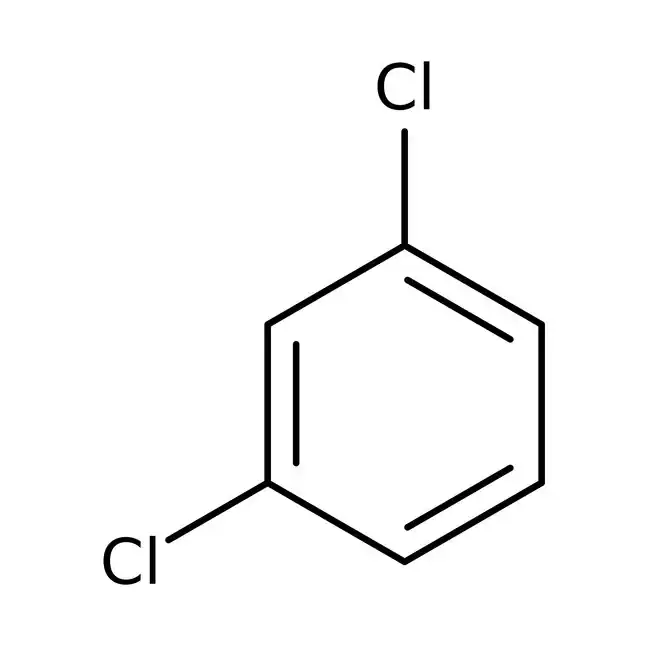
1,3-Dichlorobenzene
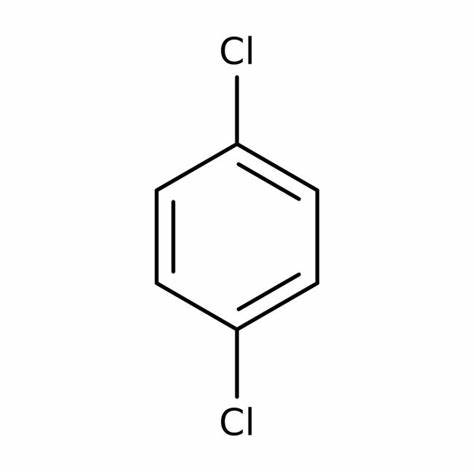
1,4-Dichlorobenzene
Conclusion: Based on this, the ¹³C NMR spectrum shown is produced by 1,3-dichlorobenzene.
Advantages of ¹³C NMR over ¹H NMR
One of the main advantages of ¹³C NMR over ¹H NMR is the wider range of the spectrum. Carbon signals resonate from 0 to 220 ppm relative to the TMS standard, while proton signals are limited to 0 to 12 ppm. This broader range in ¹³C NMR means that carbon signals rarely overlap, allowing us to clearly distinguish separate peaks for each carbon, even in larger molecules with carbons in similar environments.
For example, in the ¹H NMR spectrum of 1-heptanol, only the signals for the alcohol proton (Ha) and the two protons on the neighboring carbon (Hb) are straightforward to interpret. The other proton signals overlap, making detailed analysis challenging.
Summary
¹³C NMR Spectroscopy provides essential information about the carbon framework of organic molecules by identifying distinct carbon environments and their chemical shifts. By analyzing the number of peaks and their shifts, chemists can infer the structure or part structures of molecules. This method is a valuable tool for confirming molecular structures, often in conjunction with ¹H NMR and other spectroscopic techniques.
500K+ Students Use These Powerful Tools to Master ¹³C NMR For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
30 flashcards
Flashcards on ¹³C NMR
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards29 questions
Exam questions on ¹³C NMR
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on ¹³C NMR
Create custom exams across topics for better practice!
Try Chemistry exam builder21 papers
Past Papers on ¹³C NMR
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to ¹³C NMR you should explore
Discover More Revision Notes Related to ¹³C NMR to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Nuclear Magnetic Resonance Spectroscopy (A Level only)
Principles of NMR
374+ studying
180KViews96%
114 rated
Nuclear Magnetic Resonance Spectroscopy (A Level only)
¹H NMR
354+ studying
193KViews