Photo AI
Last Updated Sep 27, 2025
Energy levels and photon emission Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Energy levels and photon emission quickly and effectively.
212+ students studying
2.2.3 Energy levels and photon emission
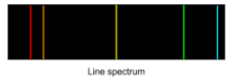
Line Spectra and Photon Emission
When light from a fluorescent tube is passed through a diffraction grating or prism, it produces a line spectrum. Each line in this spectrum represents a unique wavelength of light emitted by the tube. This line spectrum is not continuous; instead, it contains discrete wavelengths only. This is because the photon energies correspond to specific differences between energy levels in atoms, indicating that electrons in atoms can only move between discrete energy levels.
This line spectrum is evidence for quantised energy levels in atoms, as it shows that electrons can only transition between fixed energy states, emitting photons of specific wavelengths as they do so.
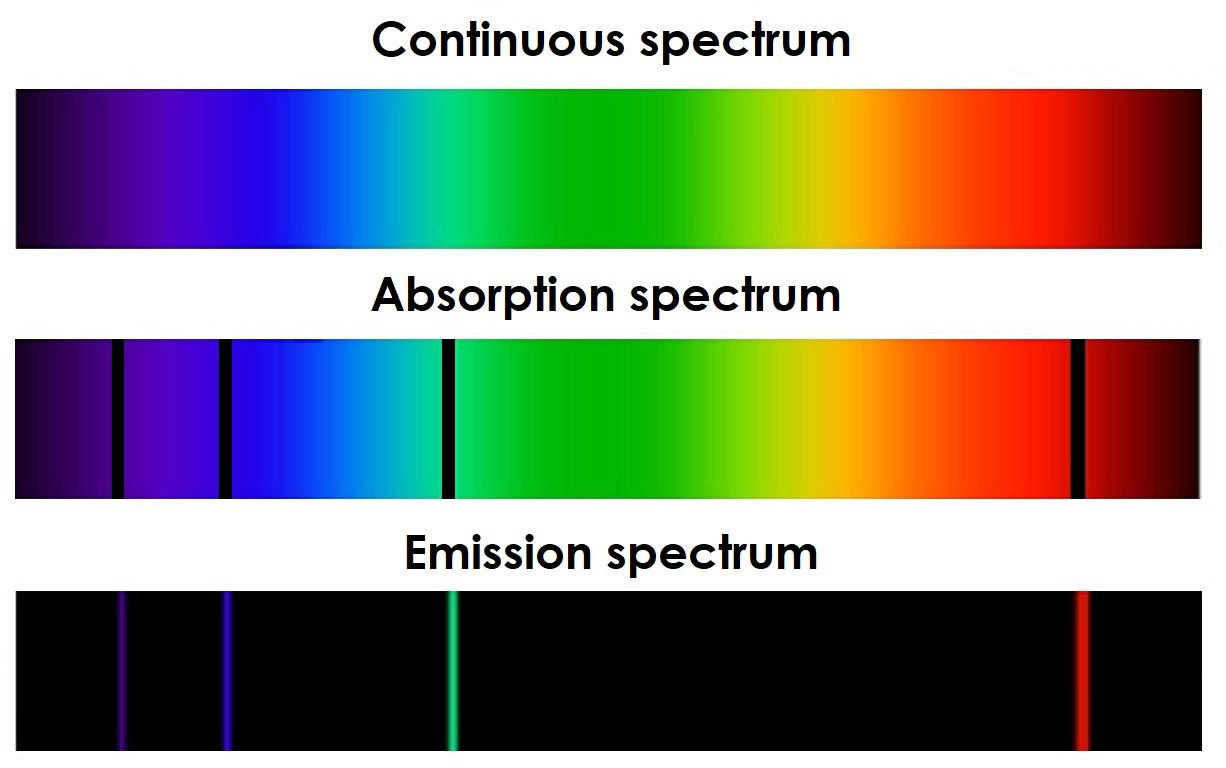
Absorption Spectra
If white light is passed through a cooled gas, it produces a line absorption spectrum. In an absorption spectrum, the continuous spectrum of all wavelengths has dark lines at specific wavelengths where light has been absorbed. These dark lines represent energy differences between the atomic energy levels. The electrons in the gas atoms absorb photons that have exactly the right energy to move to a higher energy level, leaving gaps (dark lines) in the spectrum.
Photon Energy and Energy Levels
The energy difference between two energy levels, and , is associated with the energy of the emitted or absorbed photon. This energy difference is given by:
Since the energy of a photon is also given by ( E = hf ), where ( h ) is Planck's constant and ( f ) is the frequency of the photon, we can write:
This equation allows us to calculate the frequency (and hence the wavelength) of the emitted or absorbed photon based on the difference in energy levels.
Key Points
- Line Spectrum: Produced when light from an excited gas passes through a diffraction grating, showing only discrete wavelengths.
- Absorption Spectrum: Produced when white light passes through a gas, showing dark lines where specific wavelengths are absorbed.
- Quantised Energy Levels: Electrons occupy discrete energy levels in atoms, and transitions between these levels correspond to specific photon energies.
- Photon Energy Equation: The energy of an emitted or absorbed photon corresponds to the difference between two energy levels in the atom, .
Worked Example:
- Suppose an electron transitions from an energy level of to in an atom.
- Energy Difference: .
- To convert this energy into joules, use :
- This is the energy of the photon emitted during this transition.
500K+ Students Use These Powerful Tools to Master Energy levels and photon emission For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Energy levels and photon emission
Revise key concepts with interactive flashcards.
Try Physics Flashcards4 quizzes
Quizzes on Energy levels and photon emission
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Energy levels and photon emission
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Energy levels and photon emission
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Energy levels and photon emission
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Energy levels and photon emission you should explore
Discover More Revision Notes Related to Energy levels and photon emission to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Electromagnetic radiation and quantum phenomena
The Photoelectric Effect
222+ studying
196KViews96%
114 rated
Electromagnetic radiation and quantum phenomena
Collisions of Electrons with Atoms
266+ studying
198KViews96%
114 rated
Electromagnetic radiation and quantum phenomena
Wave-particle duality
251+ studying
198KViews