Photo AI
Last Updated Sep 27, 2025
Collisions of Electrons with Atoms Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Collisions of Electrons with Atoms quickly and effectively.
359+ students studying
2.2.2 Collisions of Electrons with Atoms
Electron Energy Levels in Atoms
Electrons within an atom occupy discrete energy levels. These are fixed energy states that electrons cannot exist between. When an electron gains energy (often through collision with a free electron), it can move to a higher energy level—a process known as excitation. Alternatively, if an electron gains enough energy to completely leave the atom, the atom becomes ionised. The minimum energy required to remove an electron from the atom entirely is known as the ionisation energy.
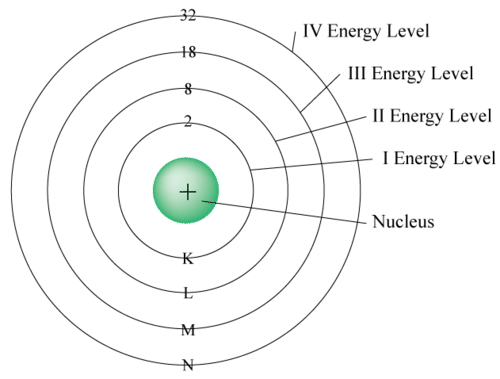
Excitation and Photon Emission
When an electron is excited to a higher energy level, it will eventually return to its ground state (original energy level). As it returns, it releases the energy it absorbed in the form of a photon. The energy of this photon corresponds to the difference between the two energy levels.
Example of Excitation:
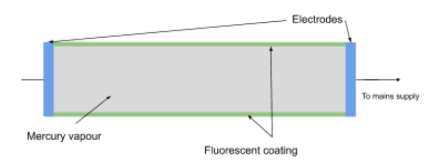
- Fluorescent tubes utilise this principle to produce visible light. In these tubes:
- Mercury vapour inside the tube is subjected to a high voltage, which accelerates free electrons through the tube.
- These free electrons collide with mercury atoms, transferring energy that can ionise or excite the mercury atoms.
- When the excited mercury atoms return to their ground state, they emit photons, most of which are in the ultraviolet (UV) range.
- The UV photons strike a fluorescent coating inside the tube, causing the coating atoms to become excited. When these atoms return to their ground state, they emit visible light.
Energy Units: The Electron Volt ()
In atomic physics, energy differences are often very small, so a unit called the electron volt (eV) is used instead of joules ().
- Definition: 1 electron volt () is the energy gained by an electron when it moves through a potential difference of volt.
- Conversion:
- =
- To convert joules to electron volts, divide the energy in joules by .
- To convert electron volts to joules, multiply the energy in by .
Key Points
- Discrete Energy Levels: Electrons exist in specific energy levels in atoms and cannot exist between these levels.
- Excitation: Electrons gain energy and move to a higher energy level, then release this energy as a photon when they return to their original level.
- Ionisation: When an electron gains enough energy to leave the atom entirely, the atom is ionised.
- Electron Volt: A unit of energy used for small-scale interactions; = .
Worked Example for Conversion:
- To find the energy in joules of an electron that gains :
500K+ Students Use These Powerful Tools to Master Collisions of Electrons with Atoms For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Collisions of Electrons with Atoms
Revise key concepts with interactive flashcards.
Try Physics Flashcards4 quizzes
Quizzes on Collisions of Electrons with Atoms
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Collisions of Electrons with Atoms
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Collisions of Electrons with Atoms
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Collisions of Electrons with Atoms
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Collisions of Electrons with Atoms you should explore
Discover More Revision Notes Related to Collisions of Electrons with Atoms to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Electromagnetic radiation and quantum phenomena
The Photoelectric Effect
464+ studying
185KViews96%
114 rated
Electromagnetic radiation and quantum phenomena
Energy levels and photon emission
317+ studying
186KViews96%
114 rated
Electromagnetic radiation and quantum phenomena
Wave-particle duality
288+ studying
200KViews