Photo AI
Last Updated Sep 27, 2025
Wave-particle duality Simplified Revision Notes for A-Level AQA Physics
Revision notes with simplified explanations to understand Wave-particle duality quickly and effectively.
368+ students studying
2.2.4 Wave-particle duality
Wave-Particle Duality of Light and Matter
Wave-particle duality describes the concept that light and particles can exhibit both wave and particle properties.
- Examples in Light:
- Wave Properties: Light demonstrates wave-like behaviours, such as diffraction and interference.
- Particle Properties: The photoelectric effect shows light acting as particles called photons.
- Wave-Particle Duality of Electrons:
- Electron Diffraction: Electrons, which are generally considered particles, also exhibit wave properties. When a beam of electrons passes through a crystal lattice or diffraction grating, it creates a diffraction pattern of concentric rings, which only waves can produce. This duality suggests that particles like electrons have wave-like characteristics, as shown through experiments involving electron diffraction.
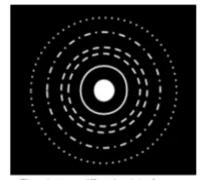
De Broglie's Hypothesis
Louis de Broglie proposed that if light (typically a wave) exhibits particle properties, then particles should also exhibit wave-like properties. He derived an equation to calculate the wavelength of a particle based on its momentum :
where:
- is Planck's constant ,
- is the mass of the particle,
- is the velocity of the particle.
Explanation of De Broglie's Equation:
- Higher Momentum (e.g., faster particles) results in a shorter wavelength, leading to less diffraction. The diffraction pattern rings become closer together.
- Lower Momentum (e.g., slower particles) results in a longer wavelength, causing more diffraction and a spread-out pattern with rings further apart. This equation connects particle motion with wave-like properties and supports the idea that particles like electrons can behave as waves under certain conditions.
Acceptance of Wave-Particle Duality
Initially, the scientific community was sceptical of the concept that particles exhibit wave properties. However, as experimental evidence accumulated, including electron diffraction and the photoelectric effect, wave-particle duality became widely accepted.
The scientific process relies on gathering experimental evidence, which must be published and peer-reviewed to be validated. This peer review ensures that findings are rigorously tested and accepted by the scientific community, contributing to the evolving understanding of concepts like wave-particle duality.
Key Points
- Wave-Particle Duality: Describes how particles and light can show both wave and particle characteristics.
- De Broglie's Wavelength Equation: Calculates the wavelength of a particle using its momentum, confirming wave-like properties in particles.
- Electron Diffraction: Experimental evidence that electrons, though particles, create diffraction patterns like waves.
- Scientific Acceptance: New concepts, like wave-particle duality, require rigorous experimental evidence and peer review to be accepted.
Worked Example:
- To find the wavelength of an electron moving at with a mass of :
500K+ Students Use These Powerful Tools to Master Wave-particle duality For their A-Level Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
40 flashcards
Flashcards on Wave-particle duality
Revise key concepts with interactive flashcards.
Try Physics Flashcards4 quizzes
Quizzes on Wave-particle duality
Test your knowledge with fun and engaging quizzes.
Try Physics Quizzes29 questions
Exam questions on Wave-particle duality
Boost your confidence with real exam questions.
Try Physics Questions27 exams created
Exam Builder on Wave-particle duality
Create custom exams across topics for better practice!
Try Physics exam builder56 papers
Past Papers on Wave-particle duality
Practice past papers to reinforce exam experience.
Try Physics Past PapersOther Revision Notes related to Wave-particle duality you should explore
Discover More Revision Notes Related to Wave-particle duality to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Electromagnetic radiation and quantum phenomena
The Photoelectric Effect
317+ studying
188KViews96%
114 rated
Electromagnetic radiation and quantum phenomena
Collisions of Electrons with Atoms
299+ studying
187KViews96%
114 rated
Electromagnetic radiation and quantum phenomena
Energy levels and photon emission
491+ studying
183KViews