Photo AI
Last Updated Sep 26, 2025
Alkenes Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Alkenes quickly and effectively.
493+ students studying
7.2.1 Alkenes
Alkenes are a type of hydrocarbon, meaning they are made up of only carbon and hydrogen atoms. They are a homologous series of unsaturated hydrocarbons that can be identified by the 'ene' at the end of their name, such as ethene.
All alkenes have carbon-to-carbon double bonds, which are represented as C=C.
The general formula for alkenes is:
- 'n' is the number of carbon atoms in the molecule
Because alkenes have a double bond between carbon atoms, they are generally more reactive than alkanes, which only have single bonds. This double bond is the functional group of alkenes and plays a key role in determining their chemical properties.
Let's look at the molecular formulae and structures of the first two alkenes:
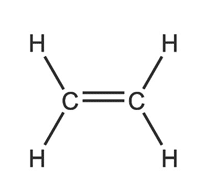
Ethene
Number of carbon atoms = 2
Molecular formula = C2H4
Structural formula:
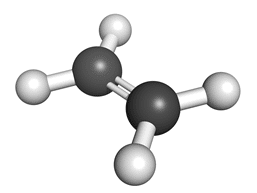
Ball + stick diagram:
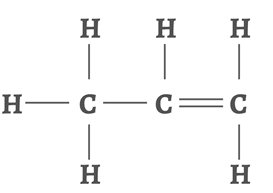
Propene
Number of carbon atoms = 3
Molecular formula = C3H6
Structural formula:
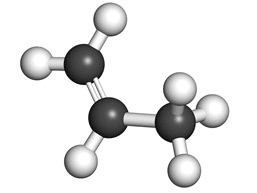
Ball + stick diagram:
As the number of carbon atoms in an alkene molecule increases, its physical properties, like boiling points, gradually change.
Alkenes have two fewer hydrogen atoms than their corresponding alkanes, making them unsaturated. The carbon-to-carbon double bond in alkenes can break open to form additional single bonds. This allows each carbon atom to form four single bonds instead of one double bond and two single bonds.
This difference in bonding is what makes alkenes more reactive than alkanes.
500K+ Students Use These Powerful Tools to Master Alkenes For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Alkenes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards29 questions
Exam questions on Alkenes
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Alkenes
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Alkenes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alkenes you should explore
Discover More Revision Notes Related to Alkenes to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reactions of Alkenes & Alcohols
Addition Reactions of Alkenes
462+ studying
189KViews