Photo AI
Last Updated Sep 26, 2025
Carboxylic Acids Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Carboxylic Acids quickly and effectively.
201+ students studying
7.2.5 Carboxylic Acids
What is a Carboxylic Acid?
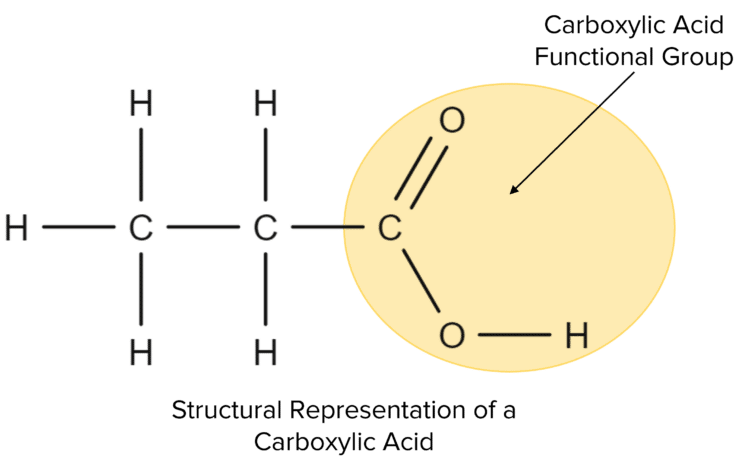
Carboxylic acids are another homologous series of molecules that can be derived from hydrocarbon molecules. All carboxylic acids have a special group of atoms called the carboxyl group (-COOH).
The Carboxylic Acid group is made up of:
- A carbon atom (C) double-bonded to an oxygen atom (O)
- The same carbon atom is also bonded to a hydroxyl group (-OH)
- Because of how these atoms are connected, the carboxyl group is always found at the end of the molecule's carbon chain.
Carboxylic acids are named based on the number of carbon atoms in their chain:
- Meth- for 1 carbon
- Eth- for 2 carbons
- Prop- for 3 carbons
- But- for 4 carbons
The name of a carboxylic acid always ends with "-anoic acid". For example, a carboxylic acid with three carbon atoms is called propanoic acid.
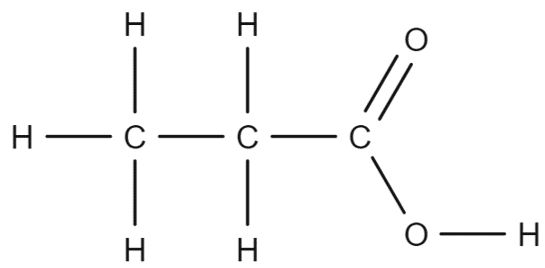
Molecular Formulae: Carboxylic Acids
The molecular formula of carboxylic acids shows how many carbon (C), hydrogen (H), and oxygen (O) atoms are in the molecule.
For instance, Propanoic acid has the formula:
- CH₃ represents the first carbon atom with three hydrogen atoms attached.
- CH₂ represents the second carbon atom with two hydrogen atoms.
- COOH represents the carboxyl group.
Reactions of Carboxylic Acids
Carboxylic acids can react in different ways, including:
- Dissolving in water: Carboxylic acids dissolve in water to form acidic solutions, meaning they have a pH of less than 7.
- Acid-base reactions: When they react with bases, like sodium carbonate, they produce salt, water, and carbon dioxide. For example:
- Propanoic acid + Sodium carbonate → Sodium propanoate + Water + Carbon dioxide
- The formula for this reaction is:
- Esterification: When carboxylic acids react with alcohols, they form esters and water. This process is called esterification.
Propanoic acid has the molecular formula CH₃CH₂COOH. Each CH group will correspond to a specific part of the molecule. The first CH₃ group corresponds to the carbon found at the end of the chain.
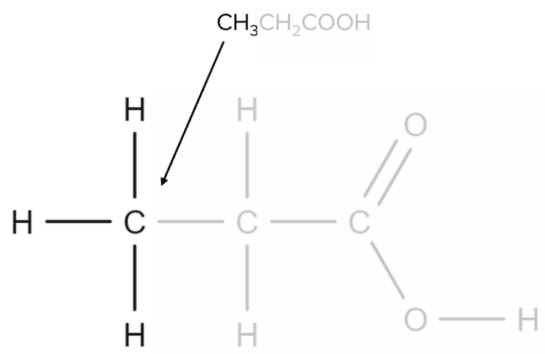
Next, the CH2CH2 group corresponds to the central carbon atom of the chain:
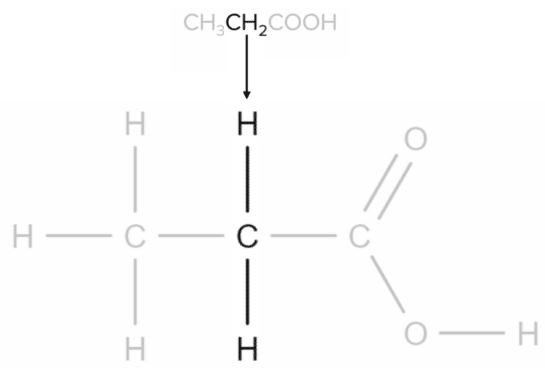
Finally, the COOHCOOH group corresponds to the carboxylic acid functional group:
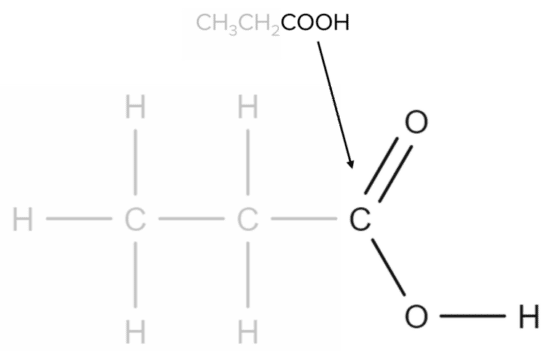
Reactions of Carboxylic Acids
Carboxylic acids undergo a range of different reactions. Similar to alcohols, carboxylic acids are soluble in water. When dissolved in water, carboxylic acids will form solutions that are acidic. These solutions can then undergo standard acid-base reactions to form salts and water.
For example, propanoic acid will react with sodium carbonate to produce a sodium salt (sodium propanoate), water, and carbon dioxide:
Carboxylic acids will also undergo reactions with alcohols. These reactions will produce new molecules called esters:
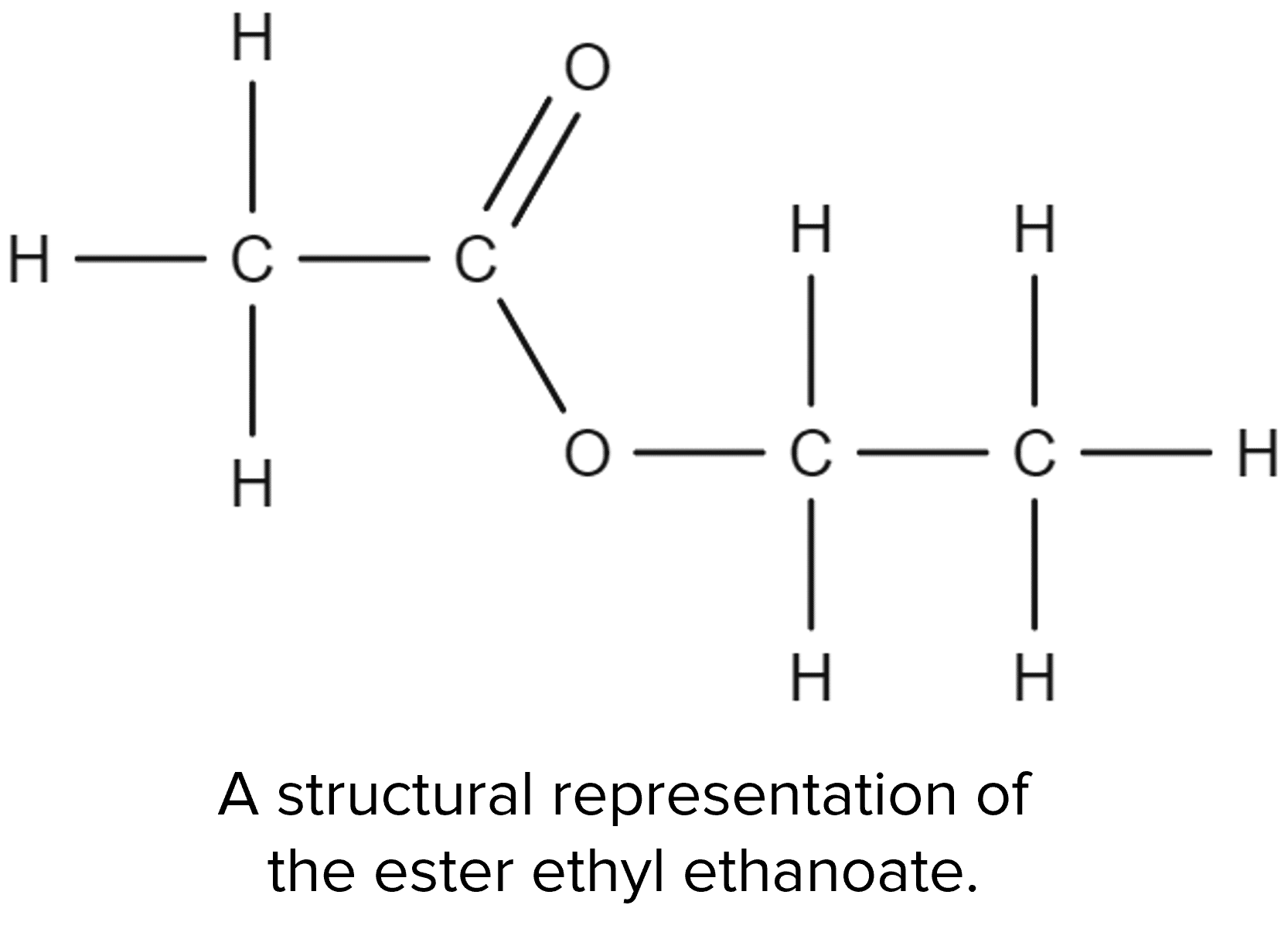
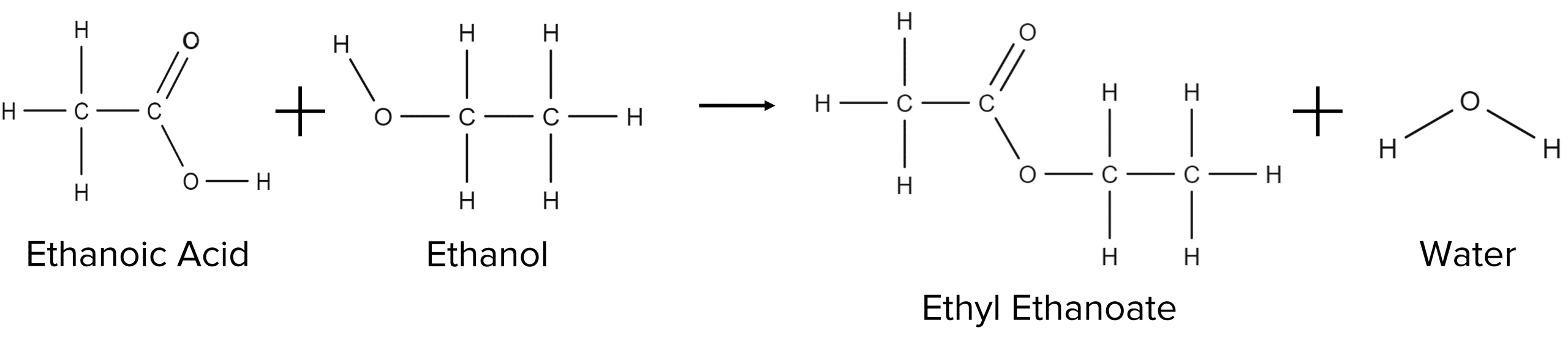
These reactions are called esterification reactions and will also produce water. Take for example the esterification of ethanoic acid by ethanol:
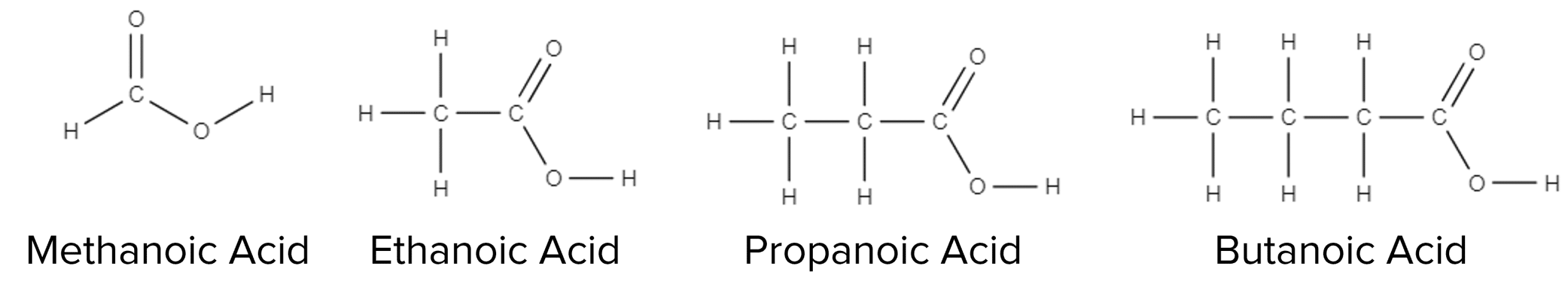
Formulae and structures of the carboxylic acids:
Methanoic acid:
Formula = CH3OH Structure:
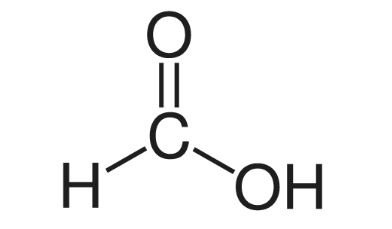
Ethanoic acid:
Formula = CH3COOH Structure:
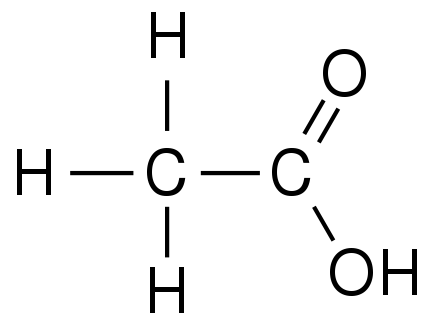
Propanoic acid:
Formula = C2H5COOH Structure:
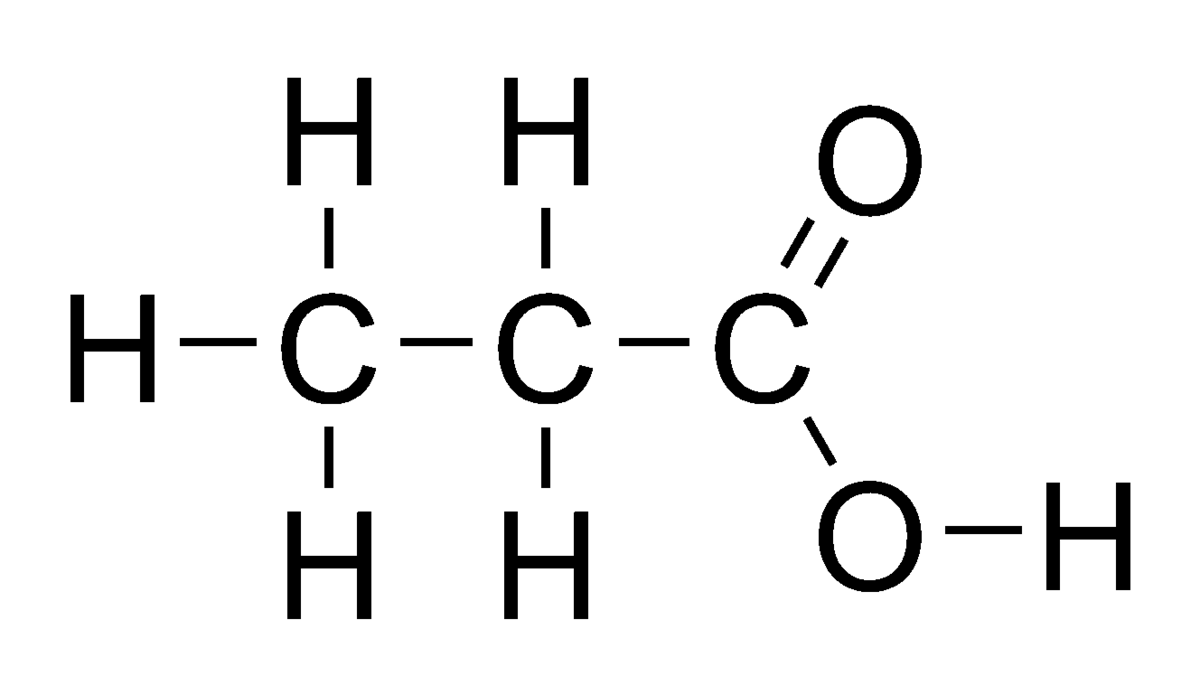
Butanoic acid:
Formula = C3H7COOH Structure:
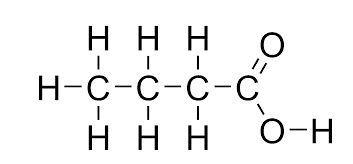
500K+ Students Use These Powerful Tools to Master Carboxylic Acids For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Carboxylic Acids
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards7 quizzes
Quizzes on Carboxylic Acids
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Carboxylic Acids
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Carboxylic Acids
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Carboxylic Acids
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Carboxylic Acids you should explore
Discover More Revision Notes Related to Carboxylic Acids to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reactions of Alkenes & Alcohols
Addition Reactions of Alkenes
229+ studying
190KViews