Photo AI
Last Updated Sep 26, 2025
Alcohols Simplified Revision Notes for GCSE AQA Chemistry
Revision notes with simplified explanations to understand Alcohols quickly and effectively.
406+ students studying
7.2.4 Alcohols
Alcohols are a group of compounds that come from hydrocarbons. They can be identified by the 'ol' ending in their names (e.g., ethanol).
The defining feature of alcohols is the hydroxyl group (OH), which influences their chemical reactions. Some alcohols have more than one hydroxyl group:
Alcohols with two hydroxyl groups are called diols.
Alcohols with three hydroxyl groups are called triols.
The general formula for alcohols is CnH2n+1OH, where 'n' represents the number of carbon atoms. For example, with four carbon atoms, the formula is C4H9OH, which is butanol.
Alcohols can also be represented by the formula R−OH, where R is a hydrocarbon chain. The number of carbon atoms in this chain can vary, which results in differences in the physical properties of alcohols. However, they share similar chemical properties.
Let's take a look at the molecular formulae and structures of the first three alcohols:
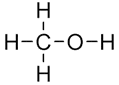
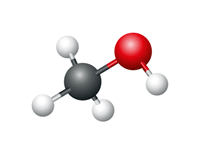
Methanol
- Number of carbon atoms = 1
- Molecular formula = CH3OH Structural formula
Ball and stick diagram
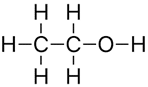
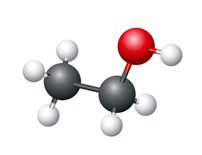
Ethanol
- Number of carbon atoms = 2
- Molecular formula = C2H5OH
Structural formula
Ball and stick diagram
Propanol
- Number of carbon atoms = 3
- Molecular formula = C3H7OH Structural formula
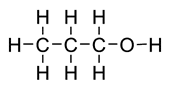
Ball and stick diagram
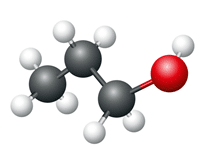
These alcohols differ from each other in the number of CH2 units in their molecular formula structures, which leads to variations in their physical properties. Nonetheless, they share the same functional group (hydroxyl) and exhibit similar chemical behaviours.
500K+ Students Use These Powerful Tools to Master Alcohols For their GCSE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
70 flashcards
Flashcards on Alcohols
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards29 questions
Exam questions on Alcohols
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Alcohols
Create custom exams across topics for better practice!
Try Chemistry exam builder26 papers
Past Papers on Alcohols
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Alcohols you should explore
Discover More Revision Notes Related to Alcohols to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Reactions of Alkenes & Alcohols
Addition Reactions of Alkenes
387+ studying
198KViews