Photo AI
Last Updated Sep 27, 2025
Electronegativity Simplified Revision Notes for Leaving Cert Chemistry
Revision notes with simplified explanations to understand Electronegativity quickly and effectively.
362+ students studying
Electronegativity
Electronegativity and Prediction of Bond Type
Electronegativity is the measure of an atom's ability to attract the shared pair of electrons in a covalent bond.
Electronegativity values can help determine whether a bond is non-polar covalent, polar covalent, or ionic:
- If the electronegativity difference is zero, the bond is non-polar covalent (pure covalent), with electrons shared equally between the atoms.
- If the electronegativity difference is between 0 and 1.7, the bond is polar covalent, where electrons are shared unequally, creating a dipole (e.g., ).
- If the electronegativity difference is greater than 1.7, the bond is ionic, where electrons are fully transferred from one atom to another (e.g., ).
Example: In , chlorine has a higher electronegativity than hydrogen, causing an unequal sharing of electrons, making the bond polar.
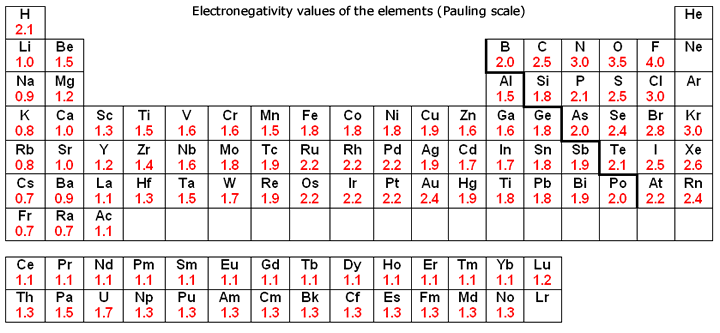
Electronegativity Values of the Elements (Pauling Scale)
500K+ Students Use These Powerful Tools to Master Electronegativity For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
120 flashcards
Flashcards on Electronegativity
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards12 quizzes
Quizzes on Electronegativity
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Electronegativity
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Electronegativity
Create custom exams across topics for better practice!
Try Chemistry exam builder115 papers
Past Papers on Electronegativity
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Electronegativity you should explore
Discover More Revision Notes Related to Electronegativity to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Chemical Bonding
Shapes of Molecules and Intermolecular Forces
414+ studying
183KViews