Photo AI
Last Updated Sep 27, 2025
Ionic Bonding Simplified Revision Notes for Leaving Cert Chemistry
Revision notes with simplified explanations to understand Ionic Bonding quickly and effectively.
242+ students studying
Ionic Bonding
What is an Ionic Bond?
An Ionic bond is an electrostatic force of attraction between oppositely charged ions when electrons are transferred from one atom to another.
An ion is formed when atoms (or groups of atoms) have either lost or gained electrons.
- A positive ion (cation) has lost one or more electrons.
- A negative ion (anion) has gained one or more electrons.
- Ions are extremely small, with cations generally being smaller than their parent atoms and anions larger.
Example of an ionic bond: Sodium Chloride
- Sodium belongs to the alkali metals in Group 1.
- Atoms of elements in this group want to lose one electron to have a stable structure.
- Chlorine belongs to the halogens in group VII and elements in this group want to gain one electron to have a stable structure.
- Therefore, in this bond, the sodium atom donates its single outer electron to chlorine.
- The atom then becomes the sodium ion (), the Cl atom then becomes the chloride ion ().
- The opposite charges attract each other.
- This force of attraction holds the ionic bond together as shown in the diagrams below.
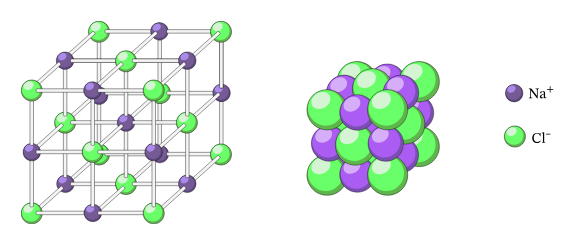
Electrostatic Force of Attraction
The electrostatic force of attraction between sodium and chloride ions is not just one-to-one but occurs in all directions around an ion.
- Therefore an ionic compound such as consists not just of a pair of ions but a network of ions held together in a three-dimensional regular and repeating pattern.
- In the case of , this lattice of ions is a cube as shown in the diagram below
The ionic crystal lattice structure of sodium chloride:
Characteristics of Ionic compounds
-
Conduct electricity when melted or in solution as their ions are free to move
-
Have high melting and boiling points due to attraction between ions
-
Most ionic substances dissolve in water
-
Exist as solids at room temperature
Everyday Use of Ionic Materials
- Salt Tablets: Used to replace salt lost during sweating.
- Water Softening: Ionic compounds are used to remove calcium and magnesium ions from hard water.
500K+ Students Use These Powerful Tools to Master Ionic Bonding For their Leaving Cert Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
120 flashcards
Flashcards on Ionic Bonding
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards12 quizzes
Quizzes on Ionic Bonding
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Ionic Bonding
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Ionic Bonding
Create custom exams across topics for better practice!
Try Chemistry exam builder115 papers
Past Papers on Ionic Bonding
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Ionic Bonding you should explore
Discover More Revision Notes Related to Ionic Bonding to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Chemical Bonding
Shapes of Molecules and Intermolecular Forces
492+ studying
197KViews