Photo AI
Last Updated Sep 24, 2025
Catalysts and Equilibrium Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Catalysts and Equilibrium quickly and effectively.
286+ students studying
Catalysts and Equilibrium
Introduction to Catalysts
Catalyst: A substance that accelerates a chemical reaction without undergoing permanent changes itself.
- Catalysts enhance reactions by reducing the activation energy, thereby increasing efficiency.
- Role of Catalysts: They expedite reactions without affecting equilibrium concentrations.
Mechanism of Action
- Catalysts offer an alternative, lower energy pathway for reactions.
- This reduction enables reactants to convert to products more readily.
Hill Climbing Analogy
- Consider a hill that decreases in height with the aid of a catalyst, facilitating the reaction's progression.
- This simplified path quickens the reaction rates.
Example: Decomposition of Hydrogen Peroxide
- Reaction:
- Explanation: Typically, this is a slow reaction, but the addition of manganese dioxide as a catalyst reduces energy requirements and significantly speeds up the process.
Equilibrium Concepts
Equilibrium State Explanation
- Equilibrium State: A dynamic condition where reactant and product concentrations remain unchanged because the rates of the forward and reverse reactions are equal.
- Role of Catalysts: They influence both the forward and reverse reaction rates equally, without shifting equilibrium positions.
Speed to Equilibrium
- Catalysts decrease the time required to reach equilibrium by accelerating both involved reactions.
- Analogy: Fast-forwarding through a film to quickly reach a specific scene does not alter the scene or its sequence.
Types of Catalysts
Homogeneous Catalysts
- Operate in the same phase as the reactants.
- Example: Acid catalysts in esterification processes.
Heterogeneous Catalysts
- Exist in a different phase than the reactants.
- Example: Nickel used in the hydrogenation of oils in industrial settings.
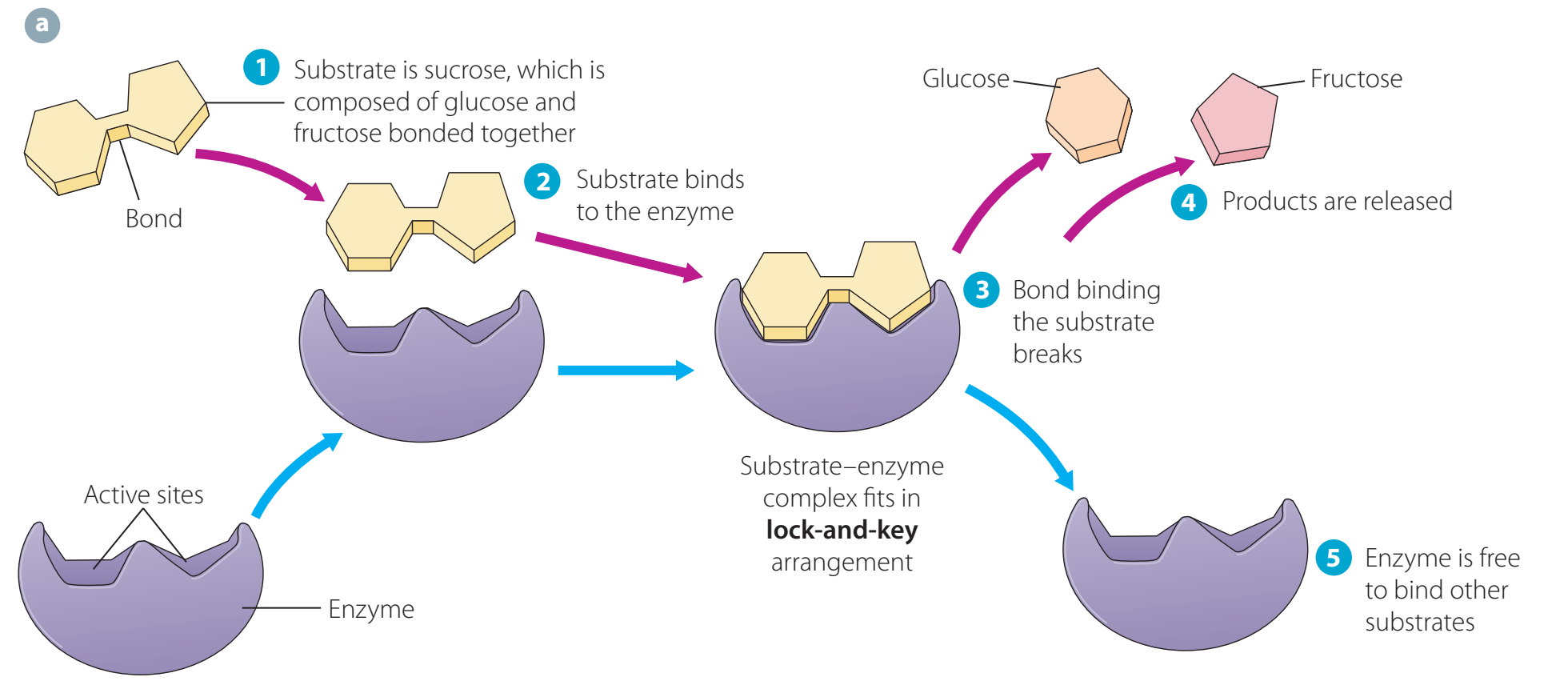
Biological Catalysts (Enzymes)
- Enzymes are crucial for biological processes and occur within metabolic pathways.
- Example: Amylase facilitates digestion by breaking down carbohydrates.
Addressing Misconceptions
- Misconception: Catalysts change equilibrium concentrations – False.
- Catalysts are not consumed in the reaction but only enhance its rate.
Remember: Catalysts increase reaction speed without altering equilibrium states.
Examples of Catalysed Equilibrium Reactions
Industrial Examples
Haber Process
- Catalyst: Iron
- Purpose: Synthesis of ammonia
- Importance: Essential for agriculture, aiding in substantial fertiliser production.
The Haber Process uses iron catalysts to expedite ammonia synthesis. While the rates are increased, the equilibrium position remains unchanged.
Contact Process
- Catalyst: Vanadium(V) oxide
- Purpose: Conversion of sulphur dioxide into sulphur trioxide
Vanadium(V) oxide greatly enhances conversion efficiency in the Contact Process.
Biological Examples
Amylase in Saliva
- Function: Decomposes starches into sugars
- Equilibrium Role: Assists in maintaining systemic energy balance
Overview of Visual Aids
Understanding catalysts in chemical reactions is enhanced by visual aids. These tools clarify the concepts of activation energy, equilibrium states, and reaction rates.
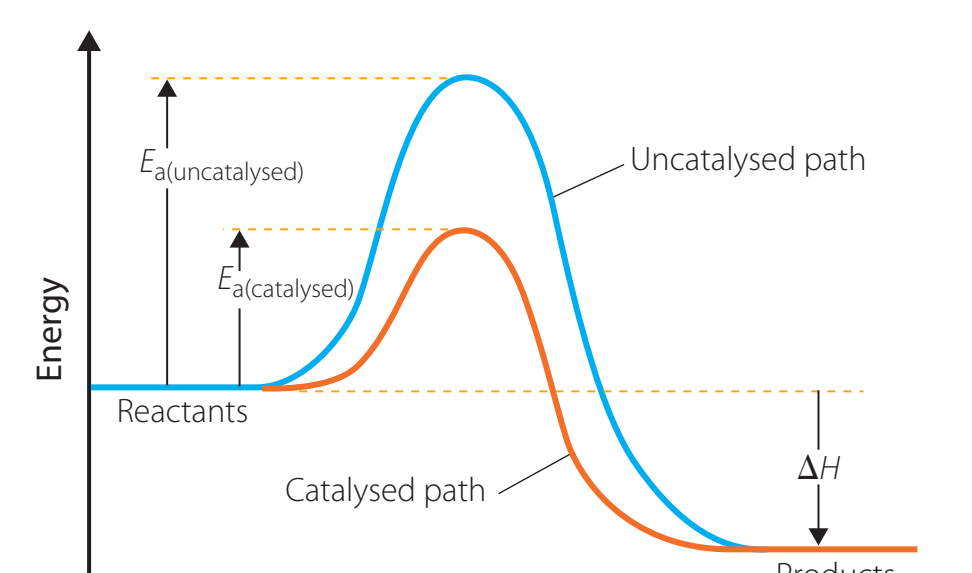
Energy Profile Diagrams

- Purpose: Demonstrates how catalysts lower activation energy, expediting reactions.
Using a catalyst results in reduced activation energy and consequently faster reaction rates.
Equilibrium Graphs
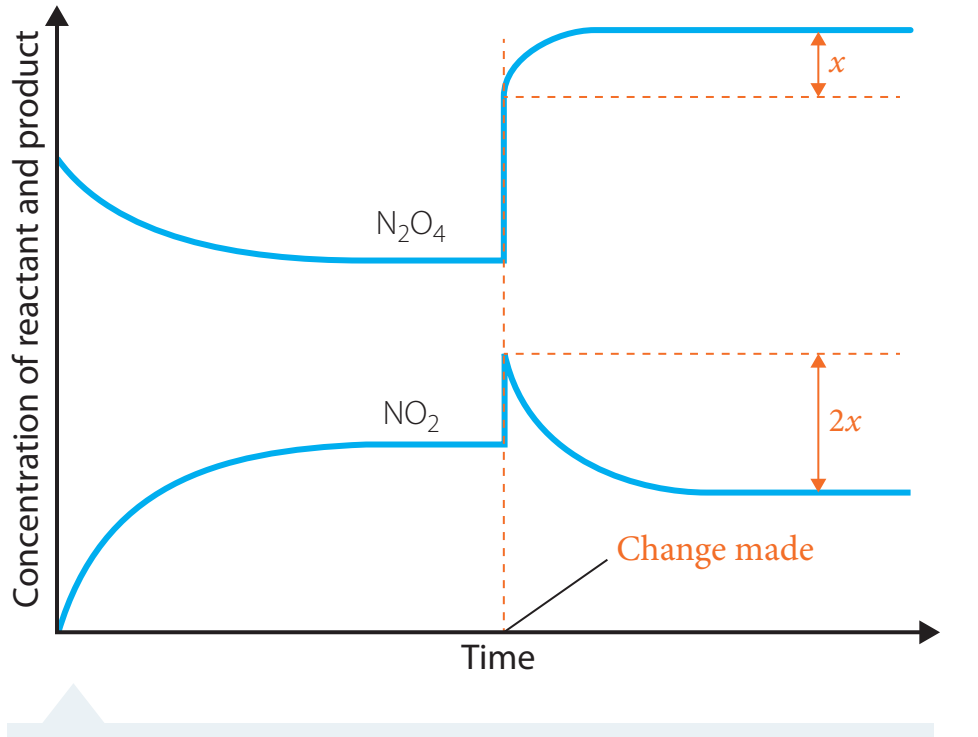
- Purpose: Illustrates quicker equilibrium attainment with catalysts without changing equilibrium concentration.
Catalysts hasten reaching equilibrium but do not alter the equilibrium composition.
Conclusion
- Catalysts effectively enhance reaction speed without influencing the inherent balance in equilibrium.
500K+ Students Use These Powerful Tools to Master Catalysts and Equilibrium For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
147 flashcards
Flashcards on Catalysts and Equilibrium
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards13 quizzes
Quizzes on Catalysts and Equilibrium
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Catalysts and Equilibrium
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Catalysts and Equilibrium
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Catalysts and Equilibrium
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Catalysts and Equilibrium you should explore
Discover More Revision Notes Related to Catalysts and Equilibrium to Deepen Your Understanding and Improve Your Mastery
