Photo AI
Last Updated Sep 24, 2025
Equilibrium Changes Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Equilibrium Changes quickly and effectively.
370+ students studying
Equilibrium Changes
This revision note addresses changes in equilibrium with a focus on concentration and partial pressure, which are crucial in chemical reactions and processes, including the Haber process.
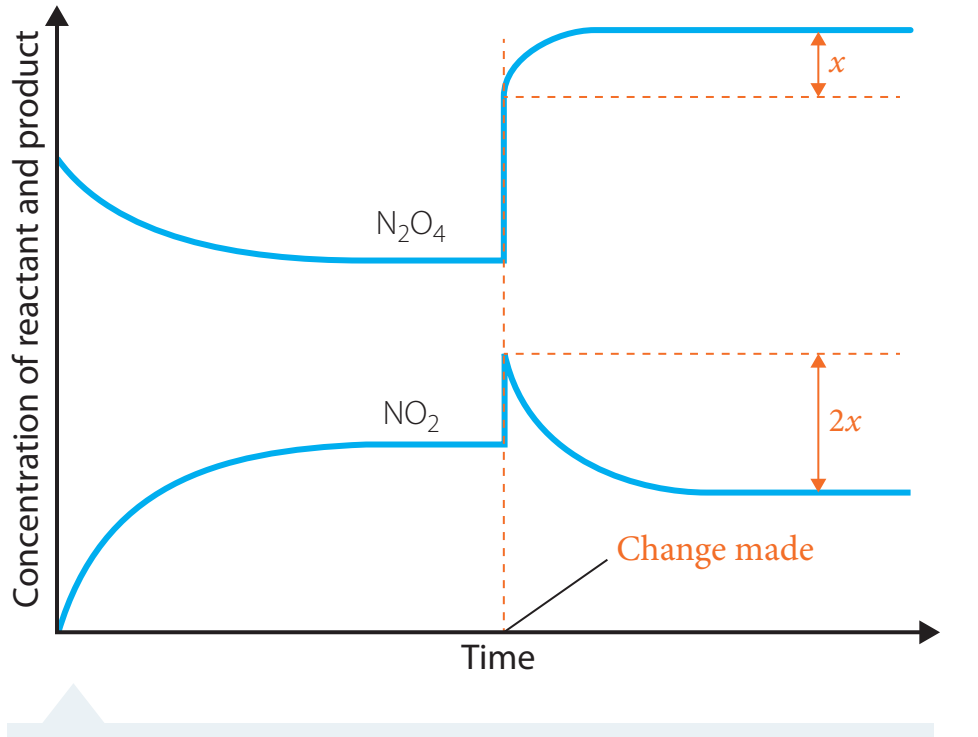
Chemical Equilibrium
- Chemical Equilibrium: This state is reached when the rates of the forward and reverse reactions are equal.
- The concentrations of reactants and products remain steady.
- It occurs within a closed system.
Dynamic Equilibrium: Equilibrium signifies a dynamic balance, rather than a static state.
- Dynamic Nature:
- This concept indicates that reactions continue to occur, continually adjusting to each other.
Equilibrium Constants
-
Equilibrium Constant :
- Defined by the concentrations of reactants and products.
- Expression:
-
Equilibrium Constant :
- Pertinent to gaseous reactions, defined in terms of partial pressures.
- Expression:
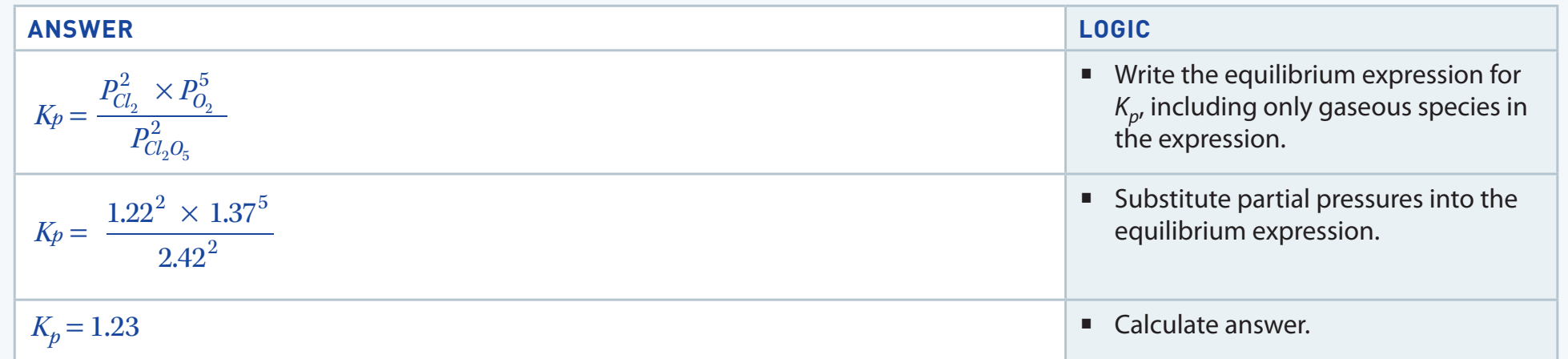
Both and remain constant with changes in concentration and pressure; however, they vary with temperature changes.
Le Chatelier's Principle
Le Chatelier's Principle: If a system at equilibrium experiences a change, it will respond to counteract the change and restore equilibrium.
- Predicts qualitative shifts when an equilibrium system is altered.
Steps to Apply Le Chatelier's Principle
-
Identify Changes:
- Concentration: Evaluate whether reactant or product levels have changed.
- Pressure: Consider how changes relate to the mole number of gases.
- Temperature: Recognise whether the reaction is endothermic or exothermic.
-
Determine Shift Direction:
- Concentration Shifts: An increase in reactants drives the equilibrium toward products.
- Example: Adding reactants shifts the balance towards the products.
- Pressure Shifts:
- Example: A reduction in pressure could shift equilibrium to the side with more gas molecules.
- Temperature Shifts: In endothermic reactions, added heat shifts the equilibrium as heat is absorbed.
- Concentration Shifts: An increase in reactants drives the equilibrium toward products.
-
Predict Outcomes:
- Diagrammatic representations are helpful for visualising molecular arrangements.
Effect of Concentration
Introduction
- Concentration: The quantity of a substance within a specified volume.
Conceptual Framework
- Equilibrium Shift:
- Changes in concentration affect the reaction's balance.
Example: Acid-Base Reaction
- Adding hydrochloric acid (HCl) to sodium acetate (CH₃COONa) causes equilibrium to shift right, compensating for increased hydrogen ion concentration.
Predicting Shifts
- An increase in concentration results in a movement toward equilibrium.
Equilibrium constants () are unaffected by concentration changes.
Effect of Pressure and Volume
-
Pressure Changes:
- Mole count: Predict movement using the number of moles.
- Haber Process: Pressure influences ammonia production due to differences in mole numbers between reactants and products.
-
Volume Changes:
- A decrease in volume increases pressure, shifting equilibrium similarly to direct pressure changes.
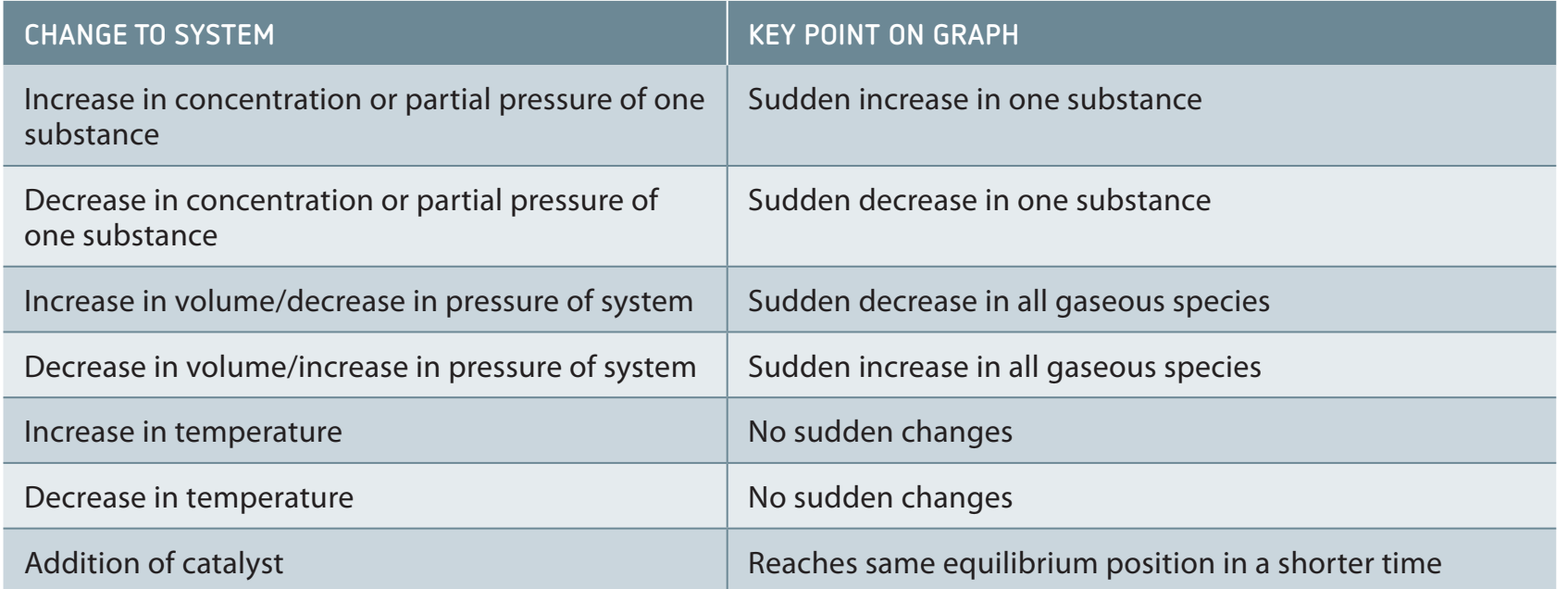
Rising pressure shifts equilibrium towards the side with fewer moles.
Applications and Worked Examples
-
Example 1: Haber Process
- Increasing the levels of nitrogen () and hydrogen pushes the equilibrium right, resulting in more ammonia production.
- This can be represented as:
- When we add more or , the system counteracts by forming more .
-
Example 2: and
- The reaction is:
- Higher pressure supports the formation of , resulting in fewer gas molecules.
- When pressure increases, the system shifts toward (1 molecule) rather than (2 molecules).
Practice Problems with Solutions
Problem 1: Simple Concentration Adjustment
- Given: In the reaction , the initial equilibrium has , , and . What happens if more is added to increase its concentration to ?
- Solution: The equilibrium will shift right to form more HI. The system will use some of the added and some to create more HI until a new equilibrium is established.
Problem 2: Volume and Pressure Change
- Given: For the reaction , what happens when the volume is halved?
- Solution: Halving the volume doubles the pressure. Since there are 4 moles of gas on the reactant side (1 + 3) and only 2 moles on the product side, the equilibrium shifts toward the product side (fewer moles of gas), producing more .
Problem 3: Temperature Changes
- Given: For the exothermic reaction , what happens when temperature increases?
- Solution: Since the reaction is exothermic (releases heat), increasing temperature will favour the reverse reaction (which absorbs heat). The equilibrium will shift left, resulting in more and , and less .
500K+ Students Use These Powerful Tools to Master Equilibrium Changes For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
147 flashcards
Flashcards on Equilibrium Changes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards13 quizzes
Quizzes on Equilibrium Changes
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Equilibrium Changes
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Equilibrium Changes
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Equilibrium Changes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Equilibrium Changes you should explore
Discover More Revision Notes Related to Equilibrium Changes to Deepen Your Understanding and Improve Your Mastery
