Photo AI
Last Updated Sep 24, 2025
Volume and Pressure Changes Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Volume and Pressure Changes quickly and effectively.
444+ students studying
Volume and Pressure Changes
Introduction
Chemical Equilibrium: Achieved when the forward and reverse reactions occur at the same rate, maintaining constant concentrations of reactants and products.
At equilibrium, the concentration of reactants and products remains stable.
- Closed System: Refers to a system where neither matter nor energy is exchanged with the surroundings, similar to a sealed container.
- Dynamic Equilibrium: Although no visible changes occur, molecular activities continue at the microscopic level.
Le Chatelier's Principle
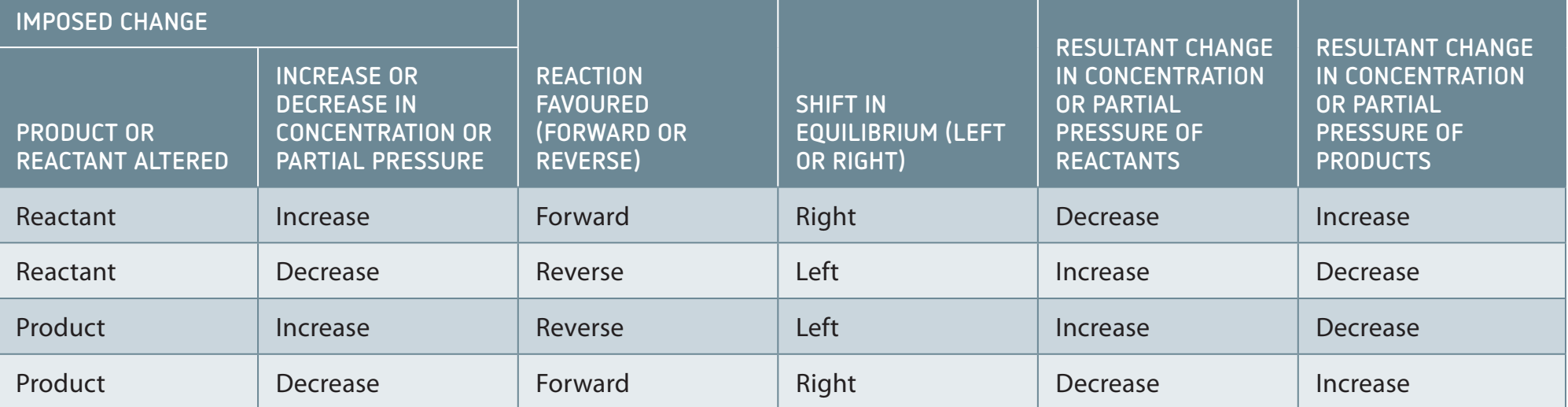
Le Chatelier's Principle: States that a system at equilibrium will adjust to counteract any changes imposed on it.
Le Chatelier's Principle explains how equilibrium is influenced by alterations in concentration, temperature, or pressure.
- Example - Pressure Change: An increase in pressure shifts the equilibrium towards fewer moles of gas.
- Example - Temperature Change: Reducing the temperature of an exothermic reaction will shift the equilibrium towards the production of more products.
Factors Affecting Chemical Equilibrium
- Concentration, Temperature, Catalysts:
- Decreasing volume induces an equilibrium shift towards fewer gas molecules.
- Catalysts accelerate reaction time but do not alter the equilibrium position.
Application of Le Chatelier's Principle: Volume and Pressure Changes
-
When Volume Decreases:
- Pressure increases.
- Equilibrium moves towards the side with fewer gas molecules.
- Example 1:
- Example 2:
-
When Volume Increases:
- Pressure decreases.
- Equilibrium shifts towards the side with a greater number of gas molecules.
Understanding Partial Pressures
- Partial Pressure: Plays a crucial role in equilibrium shifts when conditions such as volume and pressure vary.
- Contributes to the total pressure of the system.
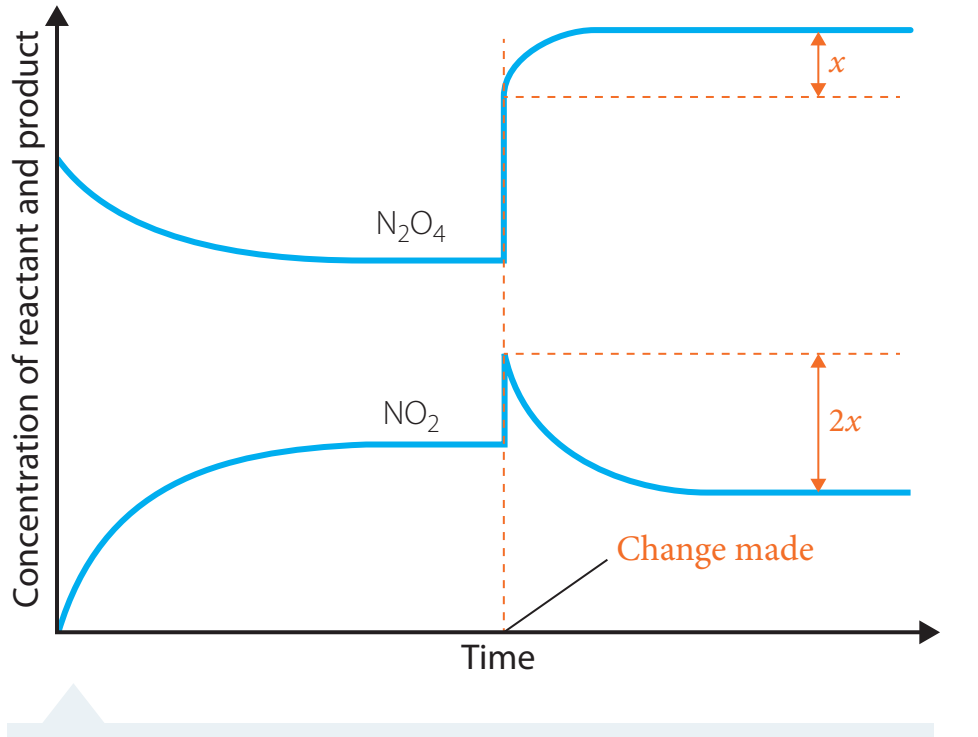
Reaction Quotient (Q)
Reaction Quotient (Q): Utilised to determine how a reaction will adjust under varying conditions.
- Q > K: Indicates a shift towards reactants.
- Q < K: Indicates a shift towards products.
- Q = K: Denotes the system is at equilibrium.
Quick Reference: Comparing Q to K assists in predicting the direction of reaction shifts.
Safety Protocols & Equipment
Emphasise safety in experiments:
- Goggles: Protect against eye injuries.
- Gloves: Shield the skin from hazards.
- Fume hoods: Ensure adequate ventilation.
Practical Implications
- Example: In ammonia synthesis, increased pressure favours the formation of fewer gas molecules.
Real-world applications illustrate the principle's relevance in industry, particularly in nitrogen fixation.

Misconceptions and Addressing Student Misunderstandings
Common Misconception: Increased pressure speeds up reactions.
Correction: Recognise the effect of molecule count on equilibrium shifts.
Worked Examples
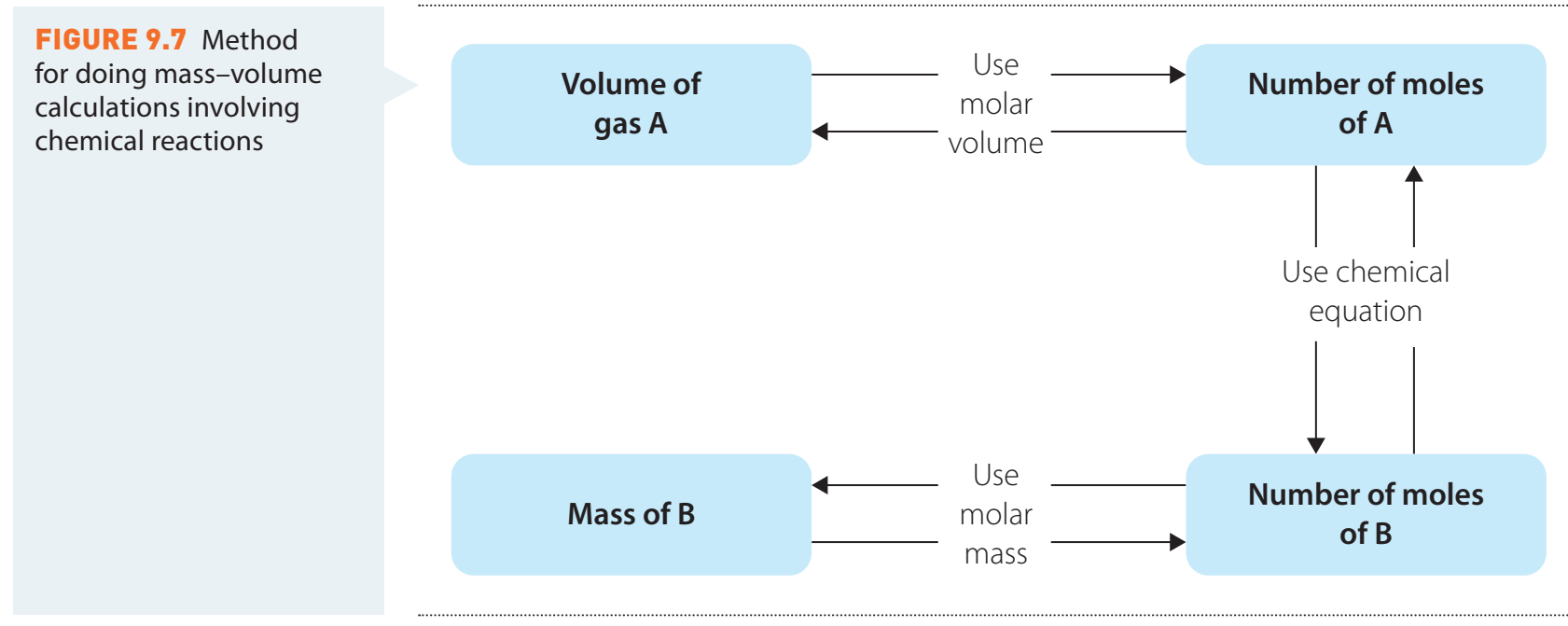
Example 1: Predict equilibrium shifts when the volume is halved for .
Solution: When the volume is halved, pressure increases. Looking at the reaction:
- Reactant side: 4 moles of gas (1 mole + 3 moles )
- Product side: 2 moles of gas (2 moles )
Since there are fewer moles of gas on the product side, the equilibrium will shift towards the formation of more to reduce pressure and counteract the change.
Example 2: Examine under doubled pressure conditions.
Solution: When pressure is doubled, the system adjusts to reduce pressure:
- Reactant side: 1 mole of gas ()
- Product side: 2 moles of gas (1 mole + 1 mole )
The equilibrium will shift towards fewer gas molecules (reactant side), increasing the concentration of and decreasing and .

Chemical Equation and Colour Change Example
- Reaction:
- Iron(III) ions interacting with thiocyanate ions can be represented as:
- Colour Change: A deep red colour indicates an increased concentration of .
Observation: A deeper red corresponds to the formation of more .

Conclusion
Understanding the effects of pressure and volume changes on chemical equilibrium provides insights into how systems respond to maintain balance according to Le Chatelier's Principle.
500K+ Students Use These Powerful Tools to Master Volume and Pressure Changes For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
147 flashcards
Flashcards on Volume and Pressure Changes
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards13 quizzes
Quizzes on Volume and Pressure Changes
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Volume and Pressure Changes
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Volume and Pressure Changes
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Volume and Pressure Changes
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Volume and Pressure Changes you should explore
Discover More Revision Notes Related to Volume and Pressure Changes to Deepen Your Understanding and Improve Your Mastery
