Photo AI
Last Updated Sep 24, 2025
Le Chatelier's Principle Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Le Chatelier's Principle quickly and effectively.
393+ students studying
Le Chatelier's Principle
Definition of Equilibrium
Equilibrium: A condition in a chemical reaction where
- The rate of the forward reaction equals that of the reverse reaction.
- The concentrations of products and reactants remain stable over time.
Equilibrium is a dynamic process, indicating that even though concentrations remain constant, molecular activities persist. This differs from a static state where no alterations occur.
Significance of Equilibrium: Without equilibrium, processes such as the Haber process and enzyme activities would be disrupted, affecting industrial and biological systems.
Characteristics of Dynamic Equilibrium
- Steady concentrations are maintained.
- Rates of forward and reverse reactions are equal.
- Functions within a closed system with no exchange of matter with the surroundings.

Graphical Representation
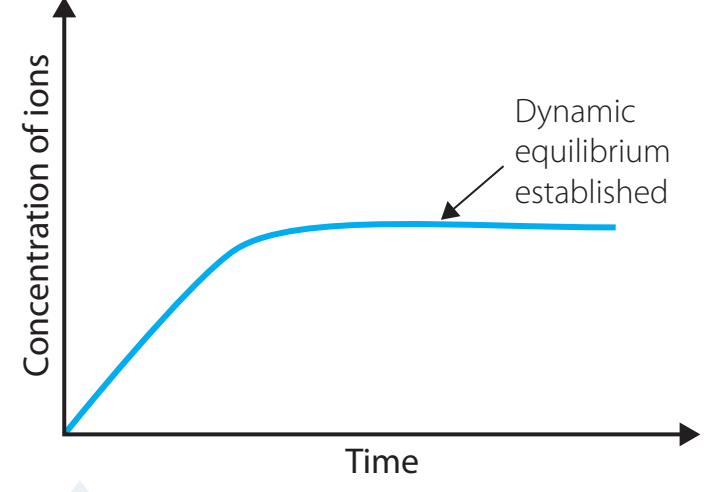
- X-axis: Time
- Y-axis: Concentration
Recognising patterns of stabilisation is essential to understanding dynamic equilibrium.
Equilibrium Constant (K)
Equilibrium Constant (K): Denotes the ratio of the concentrations of products to reactants at equilibrium:
- K varies based on the type of reaction and is crucial for predicting the equilibrium position.
- Temperature impacts K. Alterations in temperature can shift equilibrium.
Calculation Accuracy: Ensure mathematical precision for accurate results.
Introduction to Le Chatelier's Principle
Le Chatelier's Principle: "If a system at equilibrium experiences changes in concentration, temperature, or pressure, it will adjust to oppose the changes."
- Provides insight into how equilibrium adapts to changes.
- Critical for comprehending industrial applications like ammonia production.
Effects of Temperature on Equilibrium
- Endothermic Reactions: Absorb heat. An increase in temperature shifts equilibrium towards products.
- Example: Baking bread, which involves heat absorption.
- Exothermic Reactions: Release heat. An increase in temperature shifts equilibrium towards reactants.
- Example: Respiration, which involves heat release.
- Endothermic: Absorbs heat, shifting towards products.
- Exothermic: Releases heat, shifting towards reactants.
Effects of Volume and Pressure on Equilibrium
- Pressure Increase: Shifts the equilibrium toward fewer gas moles.
- Volume Decrease: Similar to increased pressure effect, shifting towards fewer moles.
- Pressure Decrease: Shifts equilibrium towards more gas moles.
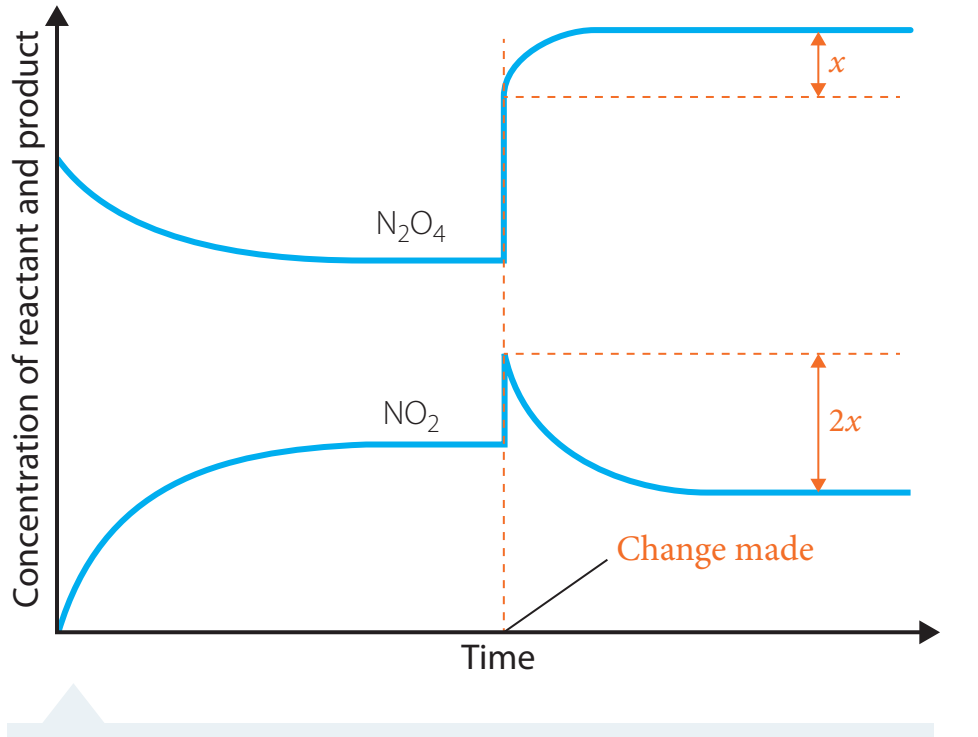
Nitrogen Dioxide/Dinitrogen Tetroxide Interaction
- Chemical Equation:
The observable colour change signifies shifts in equilibrium position.
ICE Tables
ICE Tables: Monitor initial, change, and equilibrium concentrations:
| A | B | C | |
|---|---|---|---|
| Initial | 1 | 1 | 0 |
| Change | -x | -x | +x |
| Equilibrium | 1-x | 1-x | x |
Worked Example: For a reaction A + B ⇌ C with initial concentrations of A = 1 mol/L, B = 1 mol/L, and C = 0 mol/L, and an equilibrium constant K = 4:
- Set up the ICE table as shown above
- At equilibrium: K = [C]/([A][B]) = x/((1-x)(1-x)) = 4
- Simplify: x/(1-x)² = 4
- Solve: (1-x)² = x/4
- Expand: 1-2x+x² = x/4
- Multiply both sides by 4: 4-8x+4x² = x
- Rearrange: 4x²-9x+4 = 0
- Using the quadratic formula: x = (9 ± √(81-64))/8 = (9 ± √17)/8
- Since x must be less than 1 (as it's a concentration change): x ≈ 0.61 mol/L
Therefore, at equilibrium [A] = [B] = 0.39 mol/L and [C] = 0.61 mol/L
For quadratic equations, utilise the formula:
Graphical Analysis
These graphs illustrate how reactant and product concentrations evolve over time until equilibrium is achieved.
- Components:
- X-axis: Time
- Y-axis: Concentration
Safety Considerations
- Always use gloves, goggles, and a lab coat.
- Ensure proper ventilation in the laboratory.
Safety First: Continuously prioritise protective measures during experiments.
500K+ Students Use These Powerful Tools to Master Le Chatelier's Principle For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
147 flashcards
Flashcards on Le Chatelier's Principle
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards13 quizzes
Quizzes on Le Chatelier's Principle
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes29 questions
Exam questions on Le Chatelier's Principle
Boost your confidence with real exam questions.
Try Chemistry Questions1 exams created
Exam Builder on Le Chatelier's Principle
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Le Chatelier's Principle
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Le Chatelier's Principle you should explore
Discover More Revision Notes Related to Le Chatelier's Principle to Deepen Your Understanding and Improve Your Mastery
