Photo AI
Last Updated Sep 24, 2025
Covalent Molecular Properties Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Covalent Molecular Properties quickly and effectively.
352+ students studying
Covalent Molecular Properties
Introduction to Covalent Molecular Substances
Definition and Characteristics
- Covalent Molecular Substances: Created through covalent bonds by the sharing of valence electrons between atoms. Examples include water (H₂O), carbon dioxide (CO₂), and benzene (C₆H₆).
- Key Characteristics:
- Low Melting and Boiling Points:
- This is due to weak intermolecular forces.
- Example: Ice (H₂O) melts readily.
- Electrical Conductivity:
- Typically do not conduct electricity.
- Exception: Benzene (C₆H₆) under certain conditions.
- Non-Polar Structures:
- Many are non-polar due to uniform electron distribution.
- Example: Methane (CH₄).
- Low Melting and Boiling Points:
Covalent Molecular Substances: Created through covalent bonds wherein valence electrons are shared among atoms.
Did You Know?
Diamond, while a covalent molecular solid, possesses an exceptionally high melting point owing to its strong covalent network structure.
Comparison with Ionic and Metallic Substances
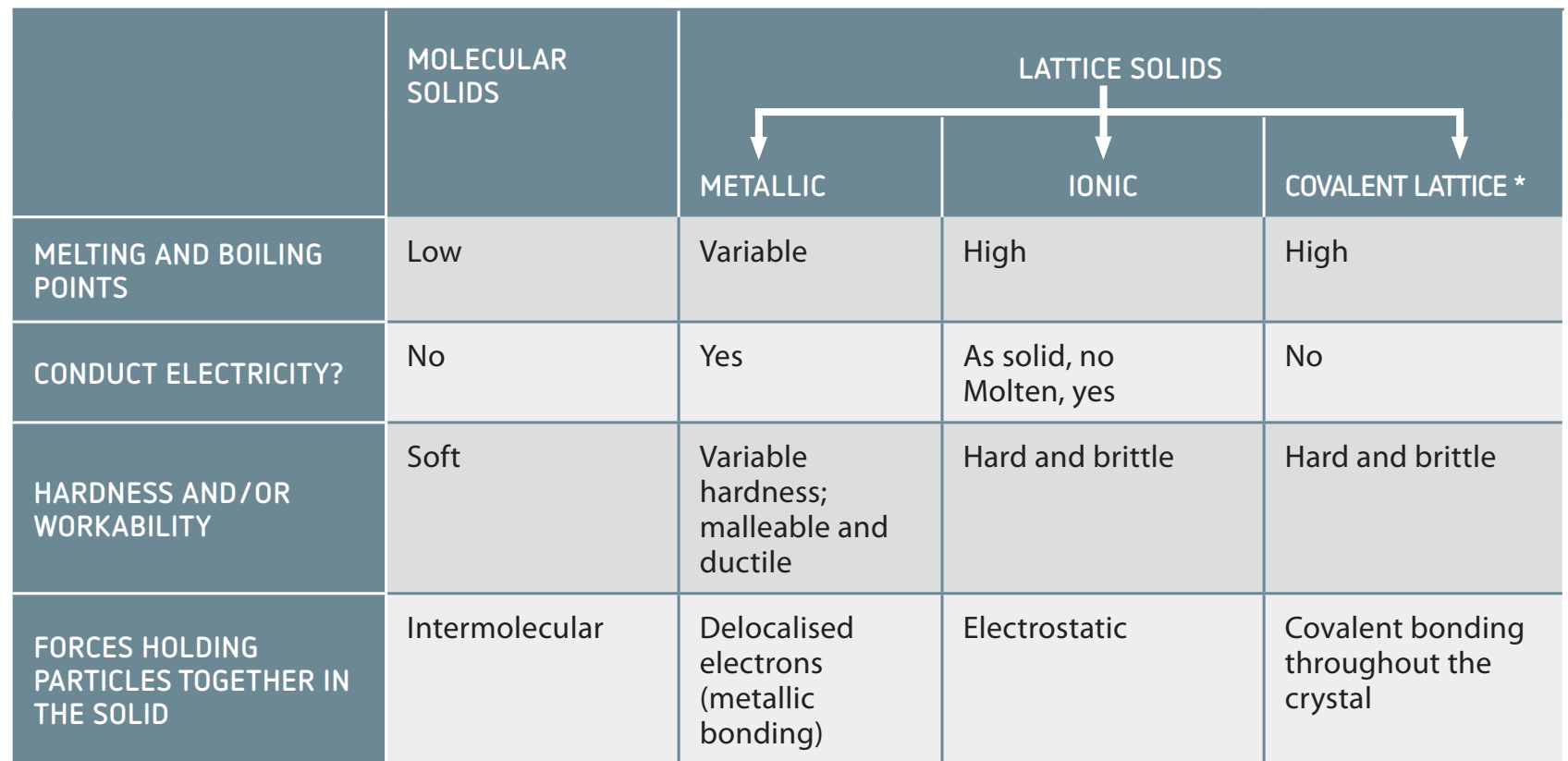
Electron Sharing and Bond Characteristics
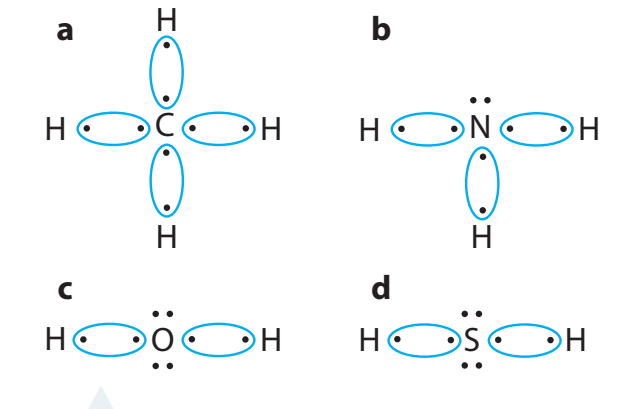
1. Electron Sharing
- Covalent Bond: The sharing of electron pairs among atoms.
- Visualised by Lewis dot structures:
- Examples: H₂ and Cl₂
2. Bond Strength and Length
-
Types of Covalent Bonds:
- Single Bond: Sharing one pair of electrons (e.g., Ethane, C₂H₆).
- Double Bond: Sharing two pairs of electrons (e.g., Ethylene, C₂H₄).
- Triple Bond: Sharing three pairs of electrons (e.g., Nitrogen, N₂).
-
Bond Strength and Stability:
- Multiple bonds confer greater stability.
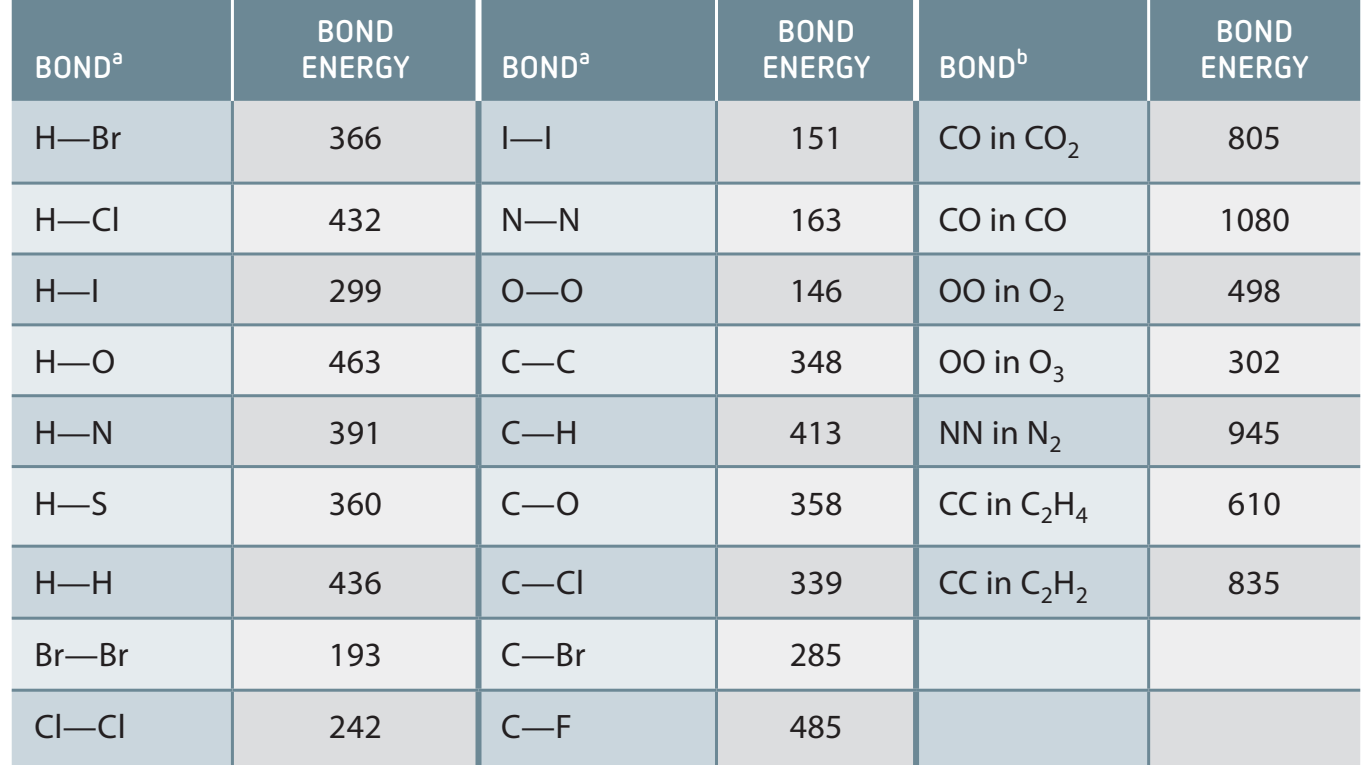
Comprehending bond length and strength is crucial for predicting reactivity and molecular stability.
VSEPR Theory and Molecular Shapes
Introduction to VSEPR Theory Application
- VSEPR Theory: Valence Shell Electron Pair Repulsion theory anticipates molecular shapes by minimising repulsion.
- Importance: Fundamental for predicting reactions and properties.
The positioning of electron pairs is pivotal to comprehending molecule shapes.
Detailed Molecular Geometry Exploration
- Key Geometries:
- Linear: Angle ~180°, Example: CO₂
- Bent (Angular): Angle <120°, Example: H₂O
- Tetrahedral: Angle 109.5°, Example: CH₄
- Trigonal Planar: Angle 120°, Example: BF₃
- Trigonal Bipyramidal: Angles 90° & 120°, Example: PCl₅
- Octahedral: Angle 90°, Example: SF₆
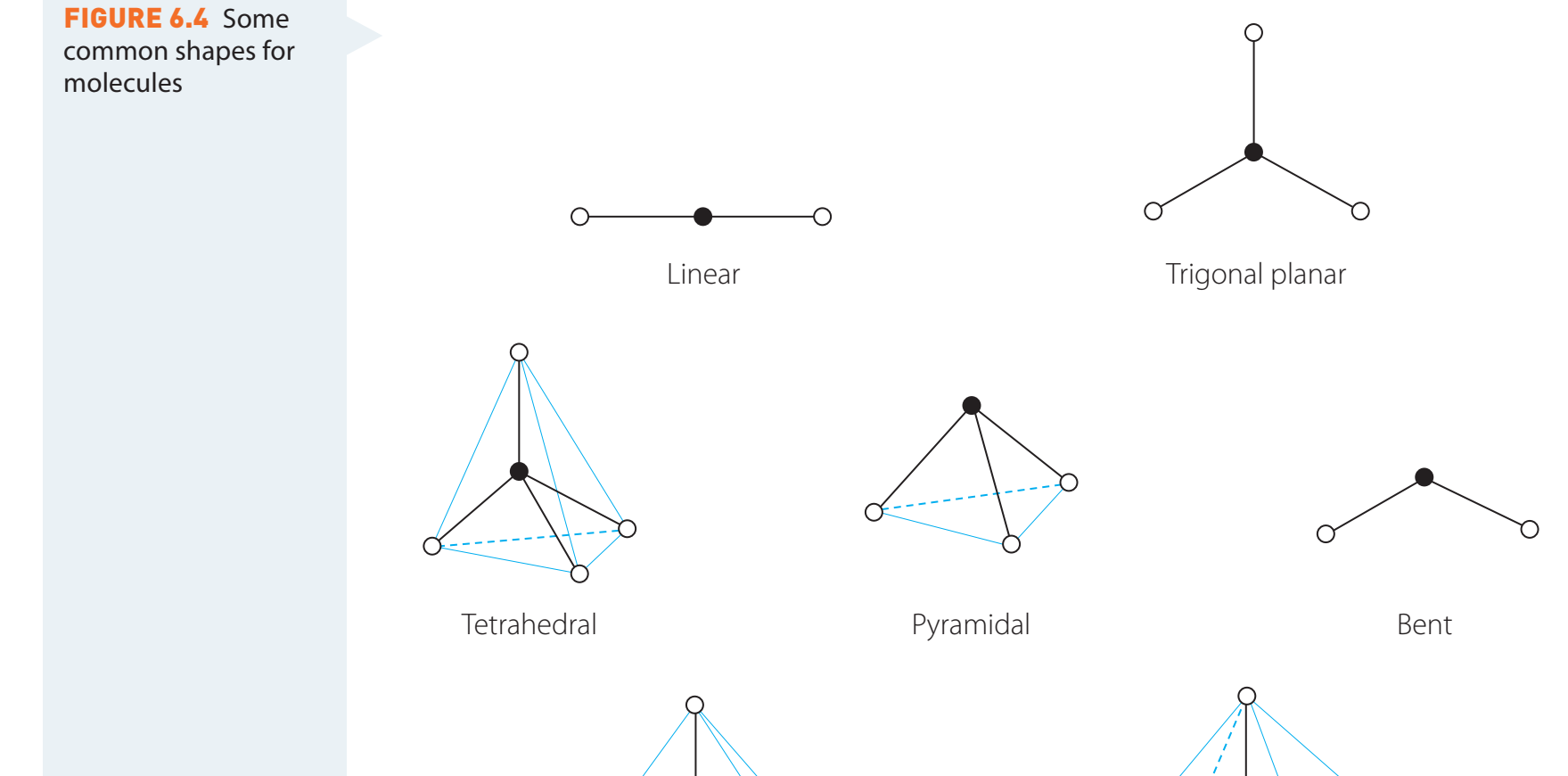
Impact of Lone Pairs and Multiple Bonds
- Lone pairs induce stronger repulsion, reducing angles in molecules such as NH₃ and H₂O.
Lone pairs and multiple bonds significantly impact molecular shapes and should not be ignored.
Physical Properties
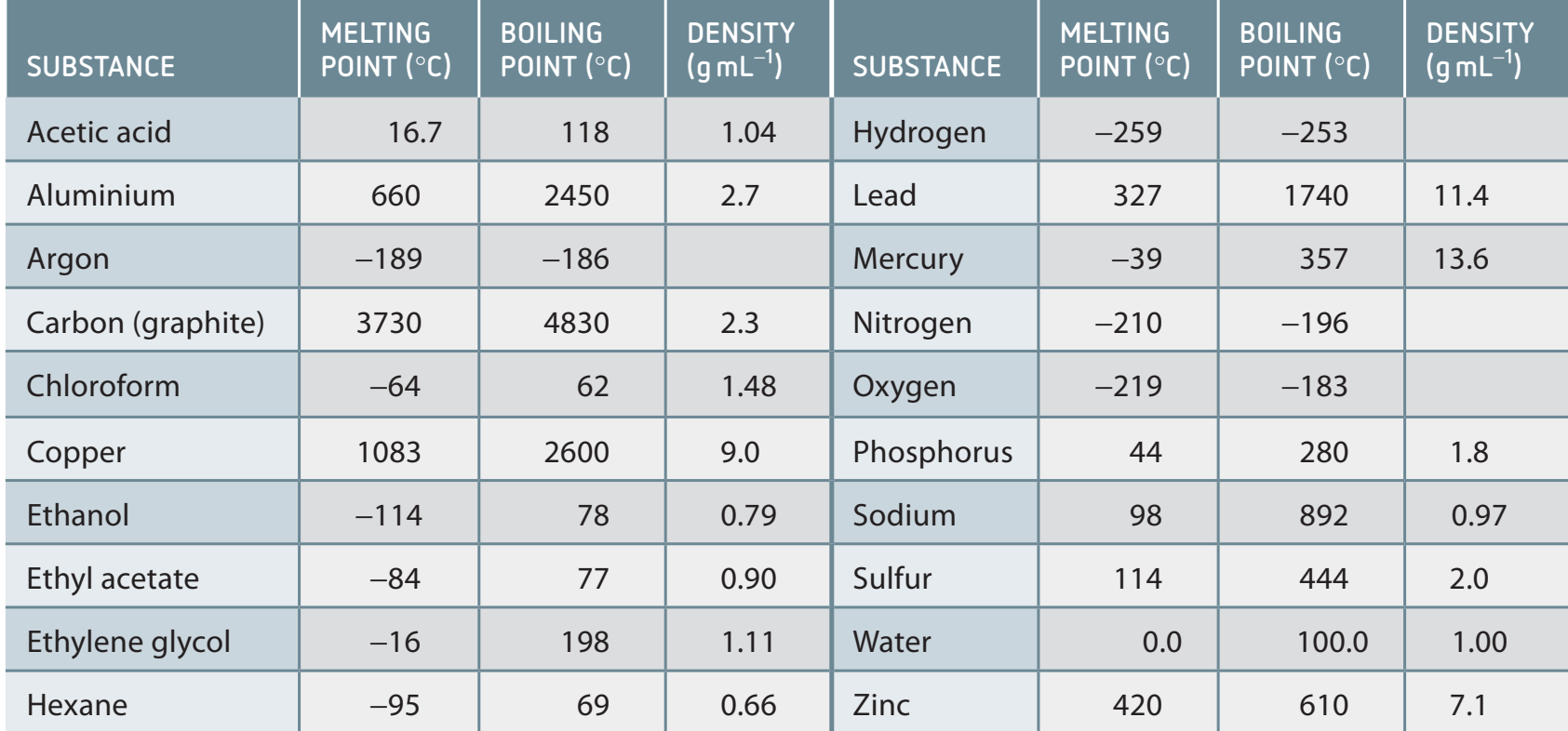
Melting and Boiling Points
- Influenced by Intermolecular Forces: Stronger forces lead to higher melting/boiling points.
- Example: Water > CO₂ due to hydrogen bonds.
Electrical Conductivity
- Generally poor due to absence of free ions or electrons.
Solutions of acids can conduct electricity when dissolved in water.
Solubility Trends
- 'Like Dissolves Like' Principle:
- Polar substances dissolve in polar solvents and vice-versa.
- Temperature Effect:
infoNote
Higher temperatures typically increase solubility of solids but decrease gas solubility.
Exam Strategies
Keywords in exams are essential:
- Predict: Provide expected outcomes based on data.
- Explain: Offer reasons supported by evidence.
Answer Organisation
- Structure responses with an introduction, discussion, and conclusion.
Utilising molecular models and digital resources is advantageous for visualisation.
Common Exam Questions
- Predict Properties: Assess melting/boiling points and solubility from structures.
- Structure Identification: Employ VSEPR models to understand geometries.
Molecular visualisation is crucial to avoid misconceptions about shape and polarity.
Worked Example: Predicting Molecular Geometry
For a molecule with formula AB₃, where A is the central atom with no lone pairs:
- Count total electron pairs around the central atom: 3 bonding pairs, 0 lone pairs
- Apply VSEPR theory to minimise repulsion
- The molecular geometry is trigonal planar with bond angles of 120°
This can be seen in molecules like BF₃, where boron forms three single bonds with fluorine atoms, resulting in a flat, triangular arrangement.
500K+ Students Use These Powerful Tools to Master Covalent Molecular Properties For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
374 flashcards
Flashcards on Covalent Molecular Properties
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards36 quizzes
Quizzes on Covalent Molecular Properties
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes11 questions
Exam questions on Covalent Molecular Properties
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Covalent Molecular Properties
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Covalent Molecular Properties
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Covalent Molecular Properties you should explore
Discover More Revision Notes Related to Covalent Molecular Properties to Deepen Your Understanding and Improve Your Mastery
