Photo AI
Last Updated Sep 24, 2025
Properties of Ionic Substances Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Properties of Ionic Substances quickly and effectively.
205+ students studying
Properties of Ionic Substances
Introduction
Ionic substances exhibit distinct properties resulting from their crystal lattice structures. These structures are responsible for the solid and granular characteristics seen in common examples such as table salt.
Central to these properties are concepts like electrostatic attraction, the energy required for bond dissociation, solubility, and brittleness. This guide will delve into these aspects to enhance understanding.
Orderly Arrangement
- 3D Crystal Lattice Structure: Ions in ionic compounds organise into a 3D lattice pattern.
- Repeating Pattern: Ensures predictability and stability.
- Minimised Repulsive Forces: The arrangement minimises repulsion and maximises attraction.
- Ionic Stability: This configuration is crucial for structural robustness.
- Significance of 3D Arrangement:
- Predictable repeating patterns are essential for stability.
- Minimising repulsion enhances stability.
- Contributes to overall ionic stability.
Explanation of Stability
- An analogy: Consider it a tightly packed jigsaw puzzle where each piece minimises gaps for enhanced stability.
Electrostatic Attraction
- Strong Electrostatic Forces: These forces result from the attraction between oppositely charged ions, crucial for maintaining lattice structural integrity.
These forces are fundamental for maintaining stability and lattice integrity.
- Analogy: Imagine the ions as a tightly packed crowd, each holding its neighbouring ions firmly, requiring substantial force to separate.
Stability and Energy
- Energy Requirement: Significant energy is needed to dissociate ionic bonds, similar to separating strong magnets.
- Comparative Energy Requirement:
- Ionic bonds necessitate more energy than covalent or metallic bonds.
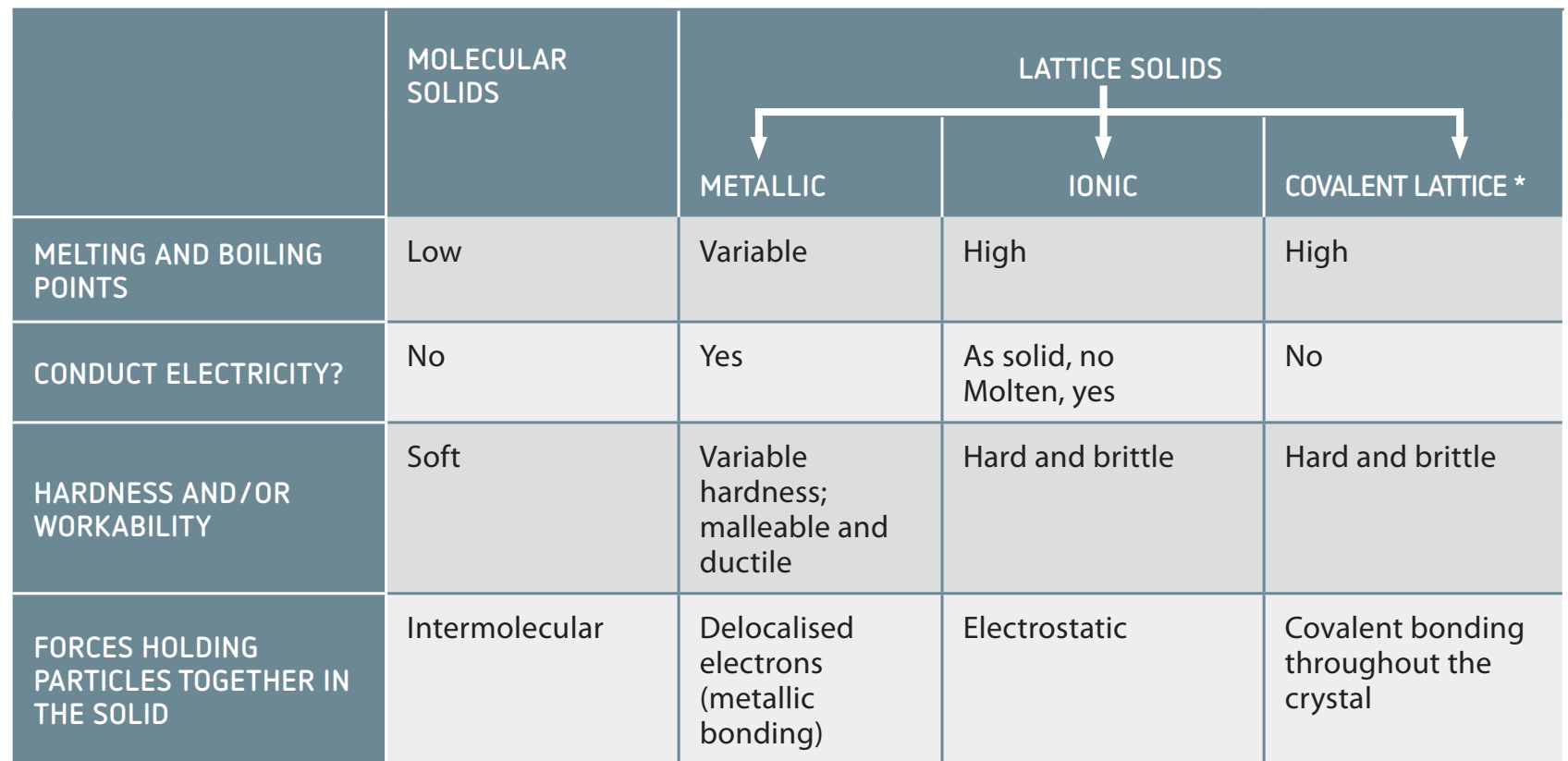
Energy Requirement: The considerable energy necessary to separate ions within a lattice.
1. Importance of Electrostatic Forces
- Role: These forces are key in stabilising ionic compounds, leading to high melting and boiling points.
Examples of Ionic Lattices
- Sodium Chloride (NaCl):
- Forms a cubic structure with dense packing.
- Alternating sodium (Na) and chloride ions (Cl) establish a stable crystal.
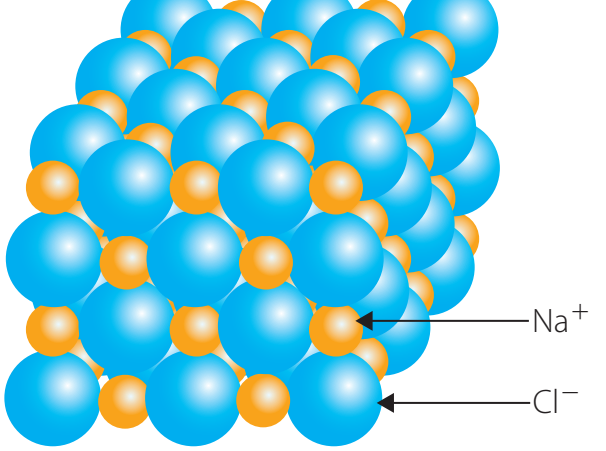
- Magnesium Oxide (MgO): Exhibits a similar lattice, contributing to strong bonds.
Conductivity
Conductivity in Solid State
- Immobility of Ions: In a solid ionic lattice, ions are held in fixed positions, hindering electrical conductivity.
Key Concept: The fixed position of ions in a solid ionic lattice inhibits conductivity.
Conductivity in Molten/Aqueous State
- Free Movement of Ions: Melting or dissolving allows ions to move freely, enhancing conductivity.
Analogy: Imagine ions in solid states like people stuck in a crowded lift, unable to move. When molten, they move freely in a spacious room.
Brittleness
Introduction to Brittleness
- Brittleness: Brittleness defines a material's tendency to fracture under stress with minimal deformation.
Brittleness: Describes the tendency to shatter without significant deformation.
Mechanism of Brittleness
- Ion Movement Under Stress: Stress induces slight ion movement, altering the structure.
- Repulsion Leading to Shattering: Like-charged ions repel under stress, causing sudden fracture.
Cleavage along Crystal Planes
- Cleavage Patterns: Occur along planes of weakness due to ion alignment.
- Analogy: Breaking a twig along its grain illustrates cleavage.
Solubility and Ion-Dipole Interaction
Ion-Dipole Interaction
- Solubility: Solubility describes the ability of a substance to dissolve in a solvent at the molecular level.
- Ion-Dipole Bonds: Polar solvents with partial charges interact with ions based on electrostatic attraction, stabilising dissolved ions.
Example: NaCl dissolves as sodium ions (Na) interact with water's oxygen and chloride ions (Cl) with hydrogen.
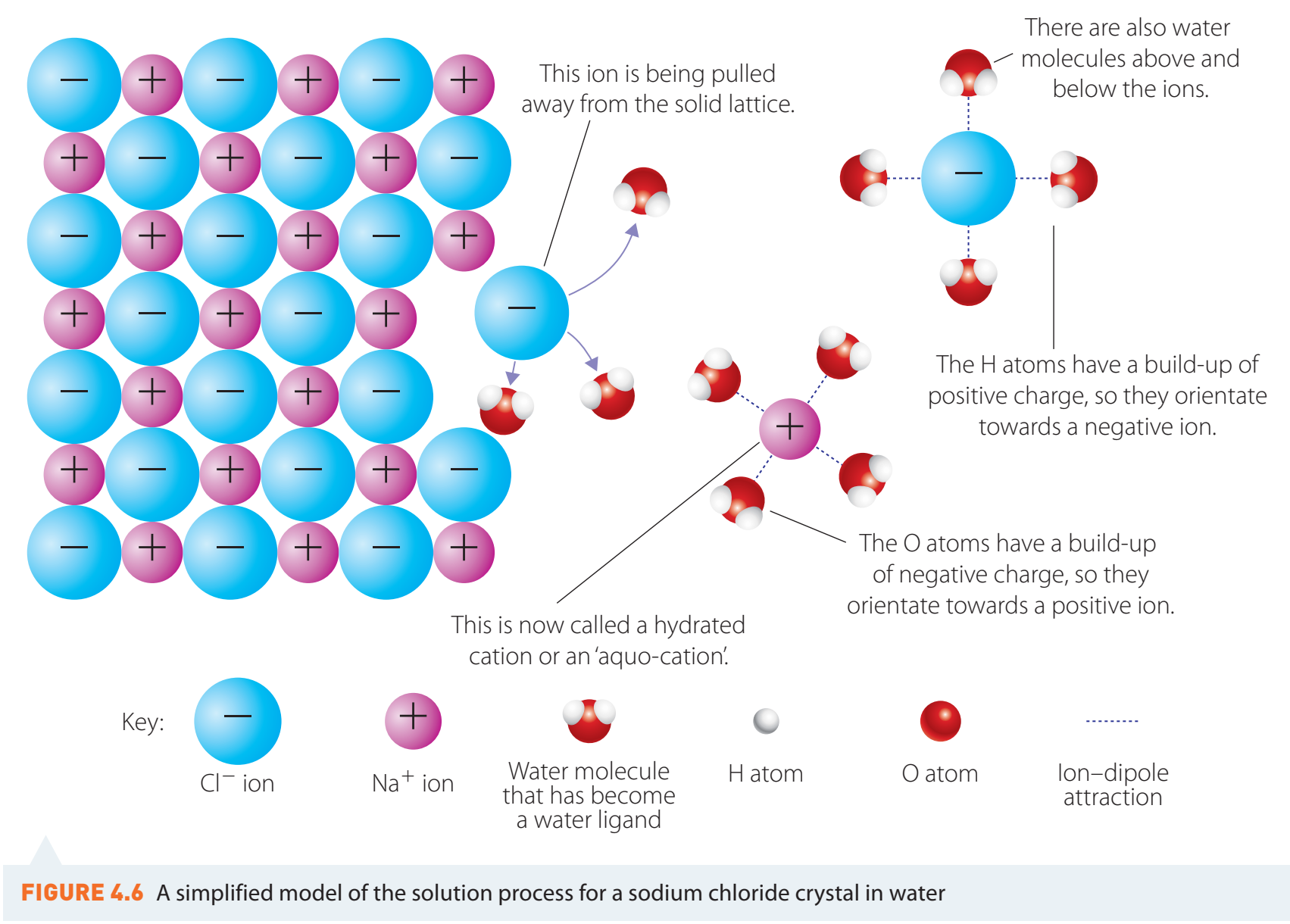
Enhancements and Highlights
- Key Concepts: Emphasise concepts like solubility and electrostatic attraction.
Fun Fact: Ever wondered why salt dissolves in water but oils do not? It is because oil lacks polar molecules to form ion-dipole interactions.
Summary of Key Points
- Electrostatic Forces: Integral for stability and high melting/boiling points.
- Energy Requirements: Indicative of significant bond strength.
- Conductivity: Depends on ionic mobility.
- Brittleness: Results from lattice structure.
- Solubility: Influenced by ion-dipole interactions in polar solvents.
500K+ Students Use These Powerful Tools to Master Properties of Ionic Substances For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
374 flashcards
Flashcards on Properties of Ionic Substances
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards36 quizzes
Quizzes on Properties of Ionic Substances
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes11 questions
Exam questions on Properties of Ionic Substances
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Properties of Ionic Substances
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Properties of Ionic Substances
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Properties of Ionic Substances you should explore
Discover More Revision Notes Related to Properties of Ionic Substances to Deepen Your Understanding and Improve Your Mastery
