Photo AI
Last Updated Sep 24, 2025
Valency and the Periodic Table Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Valency and the Periodic Table quickly and effectively.
387+ students studying
Valency and the Periodic Table
Introduction to Chemical Bonding
Definition and Central Question
Chemical Bonding: A force that binds atoms in elements and compounds. This concept elucidates the central question: What binds atoms together? It is crucial for understanding chemical reactions and structures.
Chemical Bonding: The force that binds atoms in elements and compounds.
Types of Chemical Bonds
-
Ionic Bonds:
- Formed via electrostatic attraction between ions with opposite charges.
- Example: Sodium Chloride (NaCl), where sodium transfers an electron to chlorine, resulting in stable ions.
-
Covalent Bonds:
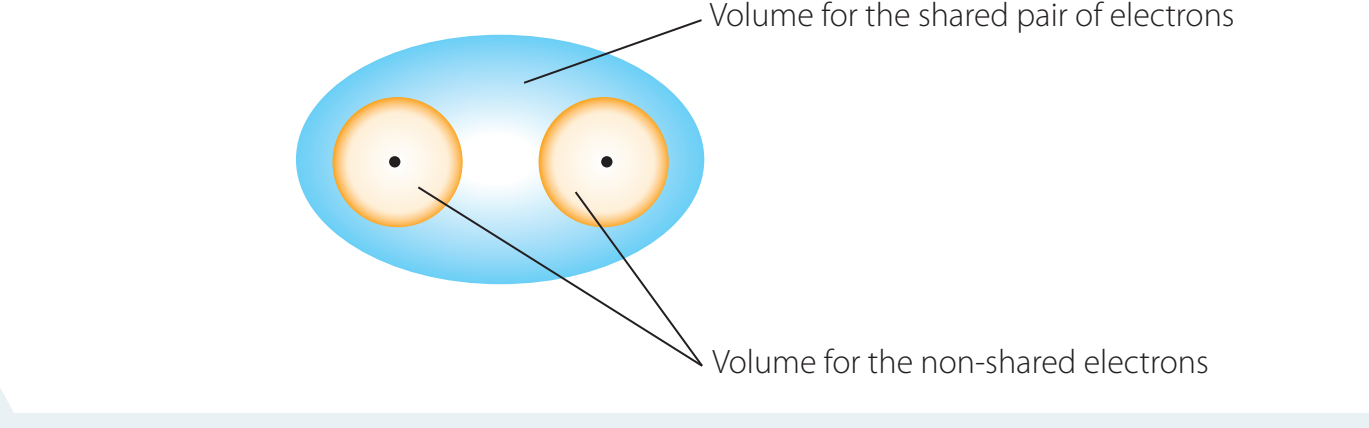
- Involve the sharing of electron pairs between atoms.
- Common examples are Hydrogen (H₂) and Water (H₂O), prevalent in nature.
-
Metallic Bonds:
- Characterised by a sea of delocalised electrons, providing structural strength to metal lattices.
Stability Through the Octet Rule
The Octet Rule explains how atoms attain a stable electron configuration similar to noble gases by gaining, losing, or sharing electrons.
Historical Perspective
-
Lavoisier: Integral to early chemical theories.
-
Mendeleev: Created the periodic table, key for predicting elements and advancing bonding theories.
-
Moseley: His study of atomic number enhanced understanding and classification of elemental bonds, modernising atomic theory.
Moseley's contributions reshaped our modern understanding of atomic bonds.
Table of Key Definitions
| Term | Definition |
|---|---|
| Chemical Bonding | The force that binds atoms together in elements and compounds. |
| Ionic Bond | Interaction via electrostatic attraction between oppositely charged ions. |
| Covalent Bond | Sharing of electron pairs between atoms. |
| Metallic Bond | Delocalised electrons within a metallic lattice structure. |
| Octet Rule | Stability akin to noble gas electron configuration. |
Valency and Electron Configuration
What is Valency and Electron Configuration?
- Valency: An atom's combining power determined by electron gain, loss, or sharing. Essential for forming compounds.
- Electron Configuration: Arrangement of electrons in an atom that defines valency.
- Illustrations of electron configuration linked to valency:
- Sodium (Na): Loses an electron - monovalent.
- Oxygen (O): Gains two electrons - divalent.
- Nitrogen (N): Shares three electrons - trivalent.
- An example of practical application, sodium chloride (NaCl), arises as sodium donates an electron to chlorine.
Role of the Periodic Table
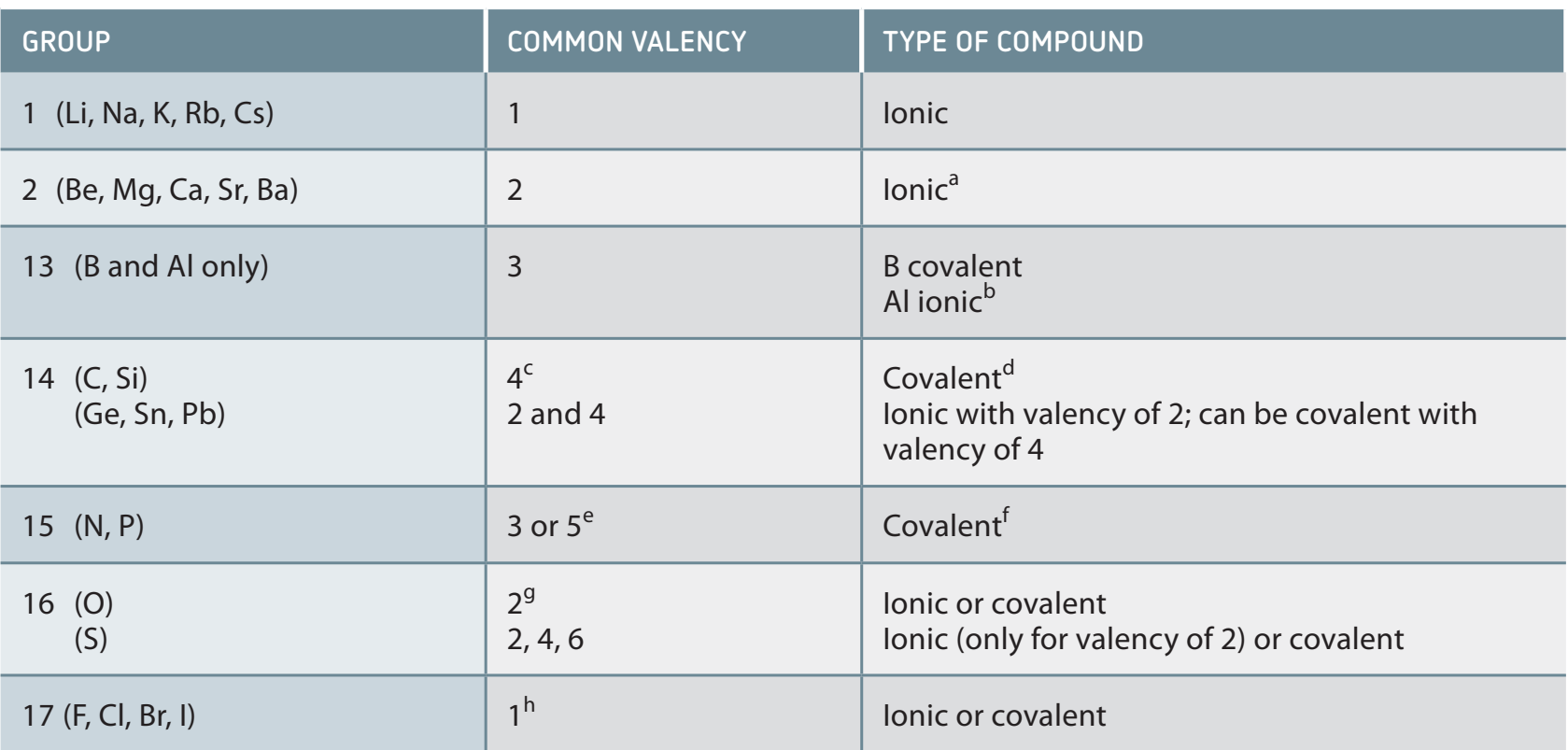
Determination of Valency Using the Periodic Table:
-
Groups:
- Elements in the same group show similar valencies due to alike electron configurations.
-
Periods:
- Across a period, valency fluctuates with increasing atomic numbers due to changing electron shells.
Historical Context and Developments
Mendeleev's and Moseley's Contributions:
-
Mendeleev's Periodic Law:
- Organised elements by atomic mass and predicted undiscovered elements based on valency.
- This arrangement informs modern chemistry by unveiling systematic valency patterns.
-
Moseley's Refinement:
- Adoption of atomic numbers refined Mendeleev's arrangements, enhancing clarity for chemical properties, including valency.
- This adaptation improves educational understanding of the periodic table.
Visualising Valency and the Periodic Table
- Efficiently using the periodic table involves examining valency patterns across groups and periods.
- The shift in the periodic table from mass-based to atomic number-based organisation enhances clarity and representation of valency trends.
The modern periodic table, arranged by atomic numbers, provides clearer insights into valency trends, aiding both academic study and real-world chemistry applications.
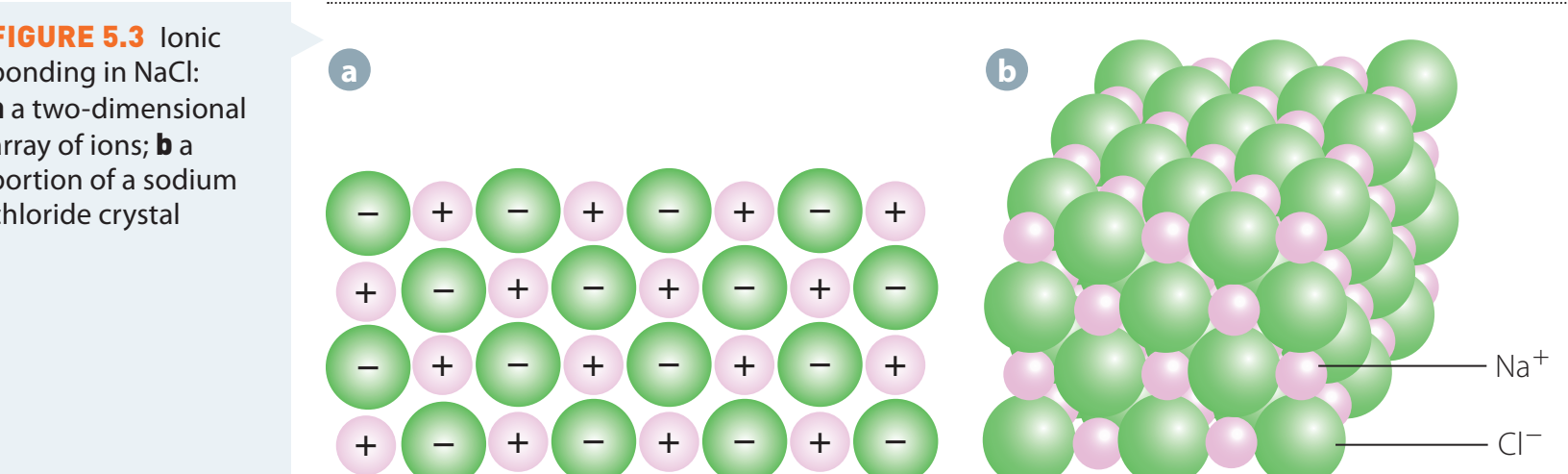
Understanding Ionic Bonding
Ionic Bonding: An electrostatic force arising from electron transfer between atoms, resulting in ions with opposite charges.
- Metals lose electrons, while nonmetals gain them to achieve full outer shells, leading to stable electron configurations.
Historical Context
- Early 20th-century chemists discovered stable compound formation through electron transfer. Contributors like Gilbert Lewis and Irving Langmuir were pivotal.
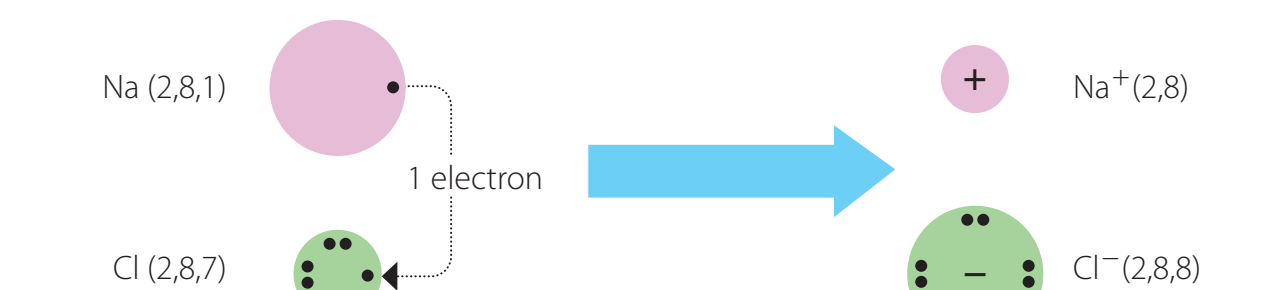
Example: Sodium Chloride (NaCl)
- Sodium (Na) loses an electron to become Na⁺.
- Chlorine (Cl) gains an electron to form Cl⁻.
- The strong electrostatic attraction results in the formation of NaCl.
Role of Valency in Ionic Compound Formation
Valency: Determines the ratio of elements in ionic compounds.
- Metals lose electrons, which are gained by nonmetals, resulting in stable compounds.
Valency: The ability of an element to bond with others to form compounds. Valency often equals the number of electrons an atom donates or receives.
Worked Example: Magnesium Oxide (MgO)
- MgO: Magnesium (Mg) loses two electrons to form Mg²⁺. Oxygen (O) gains two electrons to form O²⁻.
Example: Aluminium Oxide (Al₂O₃)
- Al₂O₃: Aluminium (Al) loses three electrons to form Al³⁺. Oxygen (O) gains two electrons to form O²⁻, requiring two Al ions and three O ions for balance.
Detailed Process for Compound Formation
- Identify Valency: Determine valency for each element in a compound.
- Align Electron Transfer: Match total electrons lost with electrons gained.
- Establish Chemical Formula: Write the formula showing balanced electron transfer.
Lewis Dot Diagrams and Electron Transfer
Lewis Dot Diagrams: Visual tools for illustrating electron transfer in ion formation.
- Diagrams show electron arrangement before and after bonding.
- Examples for NaCl, MgO, and Al₂O₃ illustrate transfer and charge.
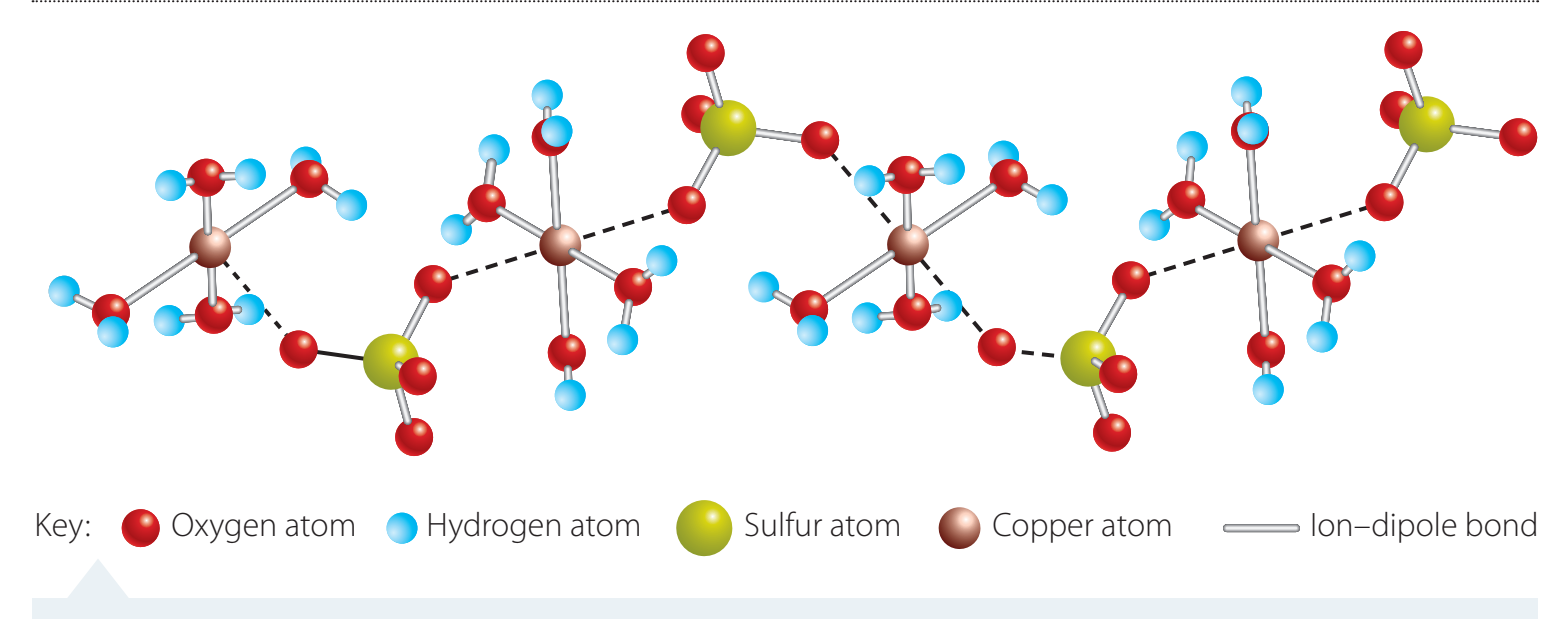
Properties of Ionic Compounds
- Ionic compounds often exhibit high melting and boiling points.
- They conduct electricity when dissolved in water due to free-moving ions.
Comparison with Covalent Compounds
- Ionic compounds feature strong electrostatic lattice structures.
- Covalent bonds are generally weaker, with lower melting points.
Key Takeaway: Mastering electron transfer and valency is essential for predicting ionic compound structures and properties.
Common Misconceptions
- Misunderstanding of Electron Sharing: In ionic bonds, electrons are transferred, not shared. This distinction is crucial for understanding stability achieved through electron exchange.
- Incomplete Octet Configurations: Ensure the octet rule is satisfied with complete electron transfer.
Reference Table for Practice
| Compound | Metal | Nonmetal | Electron Transfer | Formula |
|---|---|---|---|---|
| NaCl | Na | Cl | Na (loses 1), Cl (gains 1) | NaCl |
| MgO | Mg | O | Mg (loses 2), O (gains 2) | MgO |
| Al₂O₃ | Al | O | Al (loses 3 each), O (gains 2 each) | Al₂O₃ |
Applying these principles facilitates a clear understanding of ionic compound formation, imperative for exams and practical applications.
Introduction to Covalent Bonding
Covalent Bonding: The sharing of electron pairs between nonmetals. Atoms with similar electronegativities engage in these bonds.
Key Concepts Summary:
- Atoms with similar electronegativities engage in covalent bonding.
- Covalent bonds involve shared electron pairs.
Valency and Predicting Covalent Bonds
- Valency: Defines the number of bonds an element can form.
- It facilitates molecular structure predictions through bond determination.
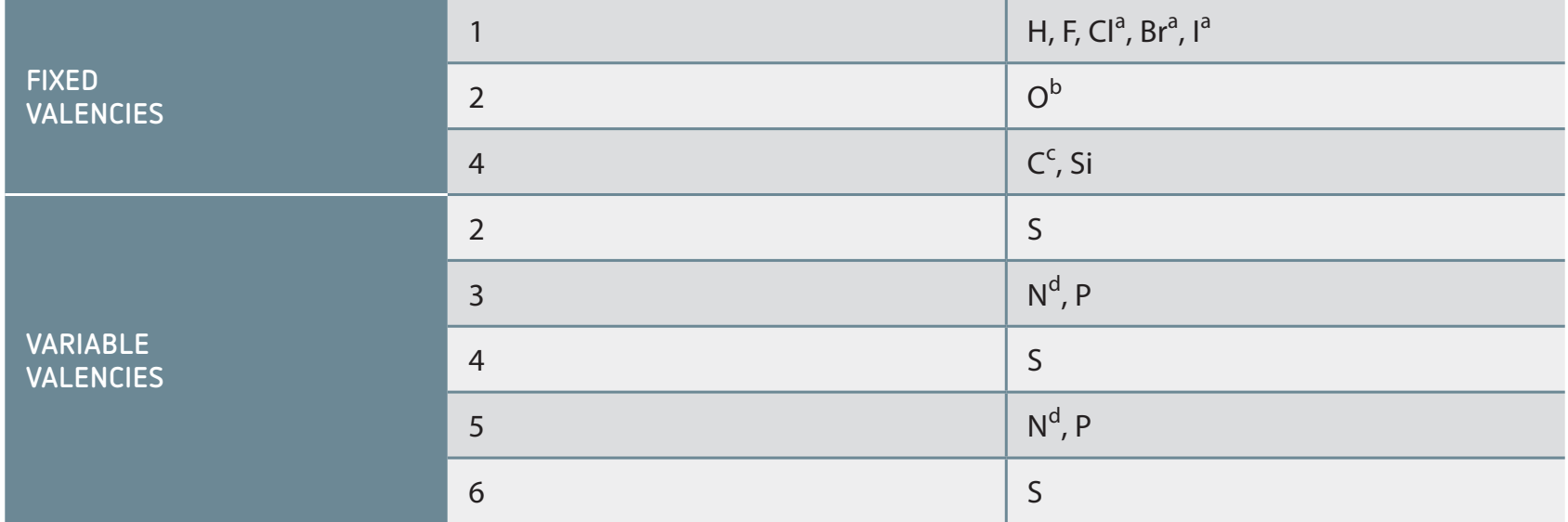
-
Practice Question: Predict the structure of a molecule where nitrogen forms three bonds.
- Solution:
- Determine Valency: Nitrogen's valency is 3, allowing three bonds.
- Consider Bonding: Nitrogen forms three sigma bonds and retains one lone pair.
- Structure Prediction: Resulting in a trigonal pyramidal shape.
- Solution:
Examples of Covalent Compounds
-
Water (H₂O): Two hydrogen atoms share electrons with one oxygen atom forming two covalent bonds.

-
Carbon Dioxide (CO₂): One carbon atom shares electrons with two oxygen atoms.
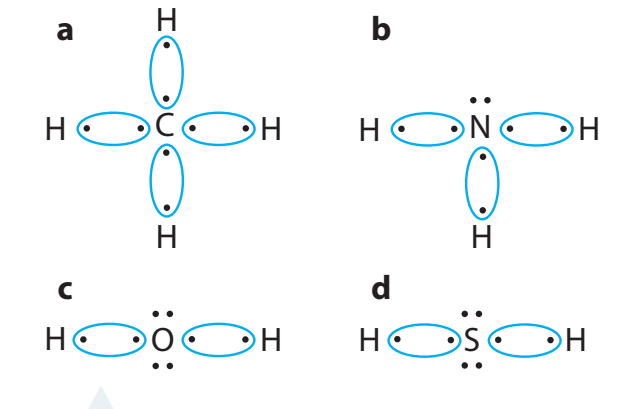
-
Methane (CH₄): One carbon atom shares electrons with four hydrogen atoms.
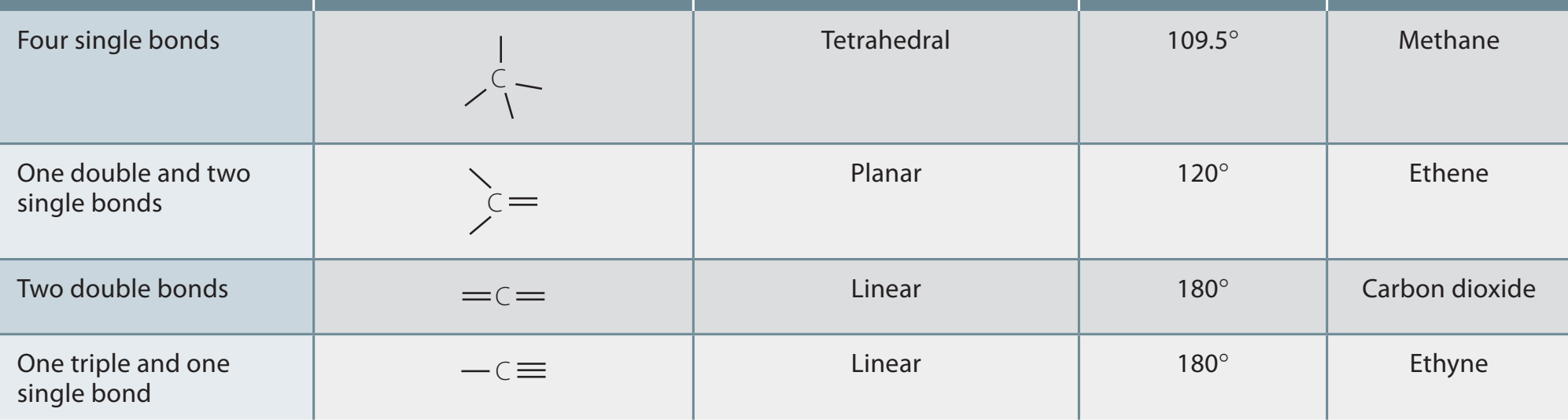
Lewis Dot Structures
- Lewis dot structures: Critical for portraying shared electron pairs.
- Steps to methodically draw them:
- Calculate the total valence electrons.
- Arrange atoms, connecting with single bonds.
- Distribute leftover electrons to fulfil octet rules.
Misconceptions Highlight: Understand the difference between lone pairs and bonding pairs clearly.
Properties of Covalent Compounds
- States: Typically gases or liquids at ambient temperature.
- Melting/Boiling Points: Generally lower than those for ionic compounds.
Misconceptions and Clarifications
- Students may confuse covalent and ionic bonds.
- Avoid relying solely on atomic numbers; consider valency charts too.
- Visual aids can effectively demonstrate electron sharing.
Example Contrast:
- Compare ionic vs. covalent by illustrating an ionic bond where electrons are transferred, not shared.
Conclusion
- Valency is integral for predicting covalent bonds.
- Crucial points to remember:
- Covalent bonds involve shared electrons.
- They typically form between nonmetals of similar electronegativity.
- Valency determines the number and type of covalent bonds an element can form.
- Covalent compounds generally appear as gases or liquids at room temperature.
Overview of Lewis Dot Diagrams
- Lewis Dot Diagrams: Represent valence electrons in atoms, fundamental for grasping molecular bonding and structure.
- Lewis Dot Diagram: A depiction showing valence electrons around an atom's symbol.
Diagramming Valency and Bond Formation
-
Steps to Create Lewis Dot Diagrams:
- Identify Valence Electrons: Count electrons in the atom's outer shell.
- Placement: Arrange dots around the element's symbol.
- Bond Formation: Utilise pairs or lone dots to suggest bonds.
-
Symbols and Notations:
- Symbols: Indicate the element and inner electrons.
- Dots: Indicate valence electrons around the symbol.
Applying to Ionic and Covalent Compounds
Ionic Compounds
- Electron Transfer:
- Electrons are fully transferred between atoms.
- Common Error: Misplacement of electron pairs in diagrams—ensure proper alignment.
Covalent Compounds
- Electron Sharing:
- Atoms share electrons rather than transferring.
- Difference: Unlike ionic transfer, covalent involves electron sharing.
Misunderstandings and Clarification
Common Misunderstandings
- Erroneously judging when to share versus transfer electrons. Key reminder: Covalent shares, ionic transfers.
Diagrammatic Variations
- Representations can differ. Ensure depiction of correct bond type—crucial for understanding.
Overview
Nomenclature: Systematic naming is vital in chemistry, ensuring clarity and global recognition, enabling chemists to communicate compound identities effectively.
Types of Compounds:
- Ionic Compounds: Formed from metals and non-metals; metals lose electrons becoming cations, while non-metals gain electrons becoming anions.
- Covalent Compounds: Created through sharing electrons between non-metals.
Systematic Rules for Ionic Compounds
Naming Cations:
- Direct Naming: Based on the element's name.
- Charge Indication: Use Roman numerals for metals with multiple ions.
- Detailed Example:
- Iron(III) Chloride (FeCl₃):
- Step 1: Identify Fe as Iron.
- Step 2: Determine charge: .
- Step 3: Name: Iron(III) to denote +3 charge.
- Iron(III) Chloride (FeCl₃):
- Detailed Example:
Naming Anions:
- '-ide' Suffix: Applied to the base element name.
- Example:
- Cl becomes Chloride (Cl⁻).
- Example:
Examples:
-
NaCl:
- Step 1: Sodium is the cation.
- Step 2: Chlorine becomes chloride.
- Final Name: Sodium Chloride.
-
Al₂O₃:
- Step 1: Aluminum as Al³⁺.
- Step 2: Oxygen becomes oxide.
- Final Name: Aluminium Oxide.
Polyatomic ions and transition metals may require specific naming conventions. Acquaintance with ions like nitrate (NO₃⁻) and ammonium (NH₄⁺) is advantageous.
Systematic Rules for Covalent Compounds
Naming System:
-
Use of Prefixes:
- Mono-, di-, tri-, etc., denote the number of atoms.
- The prefix is omitted if the first element is singular.
-
Order:
- The less electronegative element is named first.
-
'ide' Suffix:
- More electronegative gets '-ide'.
Examples:
-
CO₂:
- Step 1: Carbon comes first.
- Step 2: Utilise "di" for dioxide.
- Final Name: Carbon Dioxide.
-
P₂O₅:
- Step 1: Two phosphorus – employ "di".
- Step 2: Five oxygen – apply "pent".
- Final Name: Diphosphorus Pentoxide.
-
SF₆:
- Step 1: Sulphur first.
- Step 2: Six fluorine – use "hexa-".
- Final Name: Sulphur Hexafluoride.
Highlight on Prefixes and Suffixes
Grasping prefixes is essential for accurate naming of covalent compounds:
| Prefix | Number |
|---|---|
| mono- | 1 |
| di- | 2 |
| tri- | 3 |
| tetra- | 4 |
| penta- | 5 |
| hexa- | 6 |
Example:
- CO (Carbon Monoxide): "Mono-" denotes one oxygen atom.
Exercise Prompt:
Try these exercises for practical use:
- NH₄NO₃: Determine the naming process for Ammonium Nitrate.
- H₂O: Name as Dihydrogen Oxide.
Misconceptions and Clarifications
-
Common Mistakes:
- Confusions with polyatomic ions.
- Neglecting charge indication in transition metals.
-
Correction Strategies:
- Highlight known polyatomic ions.
- Use mnemonic aids and systematic checks.
500K+ Students Use These Powerful Tools to Master Valency and the Periodic Table For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
374 flashcards
Flashcards on Valency and the Periodic Table
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards36 quizzes
Quizzes on Valency and the Periodic Table
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes11 questions
Exam questions on Valency and the Periodic Table
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Valency and the Periodic Table
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Valency and the Periodic Table
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Valency and the Periodic Table you should explore
Discover More Revision Notes Related to Valency and the Periodic Table to Deepen Your Understanding and Improve Your Mastery
