Photo AI
Last Updated Sep 24, 2025
Carbon's Different Forms Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Carbon's Different Forms quickly and effectively.
260+ students studying
Carbon's Different Forms
Introduction to Allotropy
What is Allotropy?
Allotropy: The occurrence of elements in different forms within the same phase owing to varying atomic configurations. This concept is significant in chemistry and materials science, as it elucidates the diverse properties and applications of materials.
Visual Representation
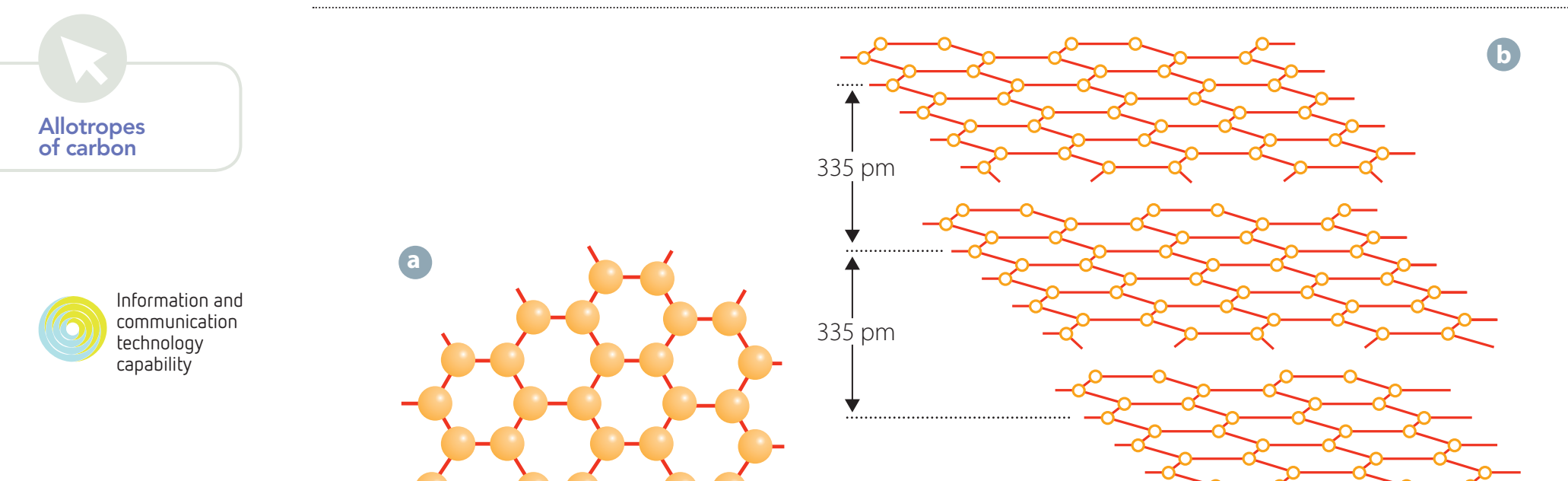
Observe the atomic structure in diamond forms a rigid, hard lattice, while graphite has layers that slide over each other. Graphene provides both strength and conductivity, making it useful for electronics such as flexible screens.
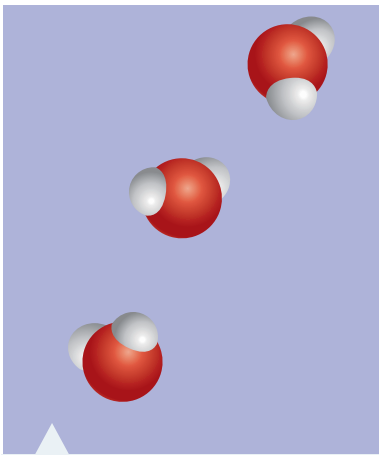
Consider the difference: Oxygen (O₂) is a diatomic molecule essential for respiration, whereas Ozone (O₃) serves as a protective layer against UV radiation, playing an essential role in maintaining ecological balance.
Common Characteristics
- Chemical Properties: Allotropes share chemical properties.
- Physical Properties Vary: Distinct structures lead to:
- Hardness: Diamond is extremely hard; graphite is soft.
- Conductivity: Graphene is conductive; diamond is not.
Historical Context
- Antoine Lavoisier (Late 18th century): Identified early differences between carbon forms.
- Smithson Tennant (1796): Proved graphite consists of carbon.
- Christian Friedrich Schönbein (1840): Discovered Ozone (O₃).
Applications
- Crucial in science and industry:
- Diamond: Used in industrial cutting tools for its hardness.
- Graphene: Utilised in flexible mobile phone screens due to its conductivity.
- Ozone: Vital for UV protection and maintaining ecological balance.
Understanding allotropy is imperative for innovations in material science, supporting the development of new technologies based on unique material properties.
Temperature and Pressure Effects
- Temperature: Influences molecular interactions, stabilising different forms.
- Pressure: Modifies structure, impacting form stability.
- Examples:
- Carbon:
- Diamond: Formed under high pressure and temperature.
- Graphite: Stable at low pressure and moderate temperature.
- Phosphorus:
- White phosphorus: Preferred in low-pressure environments.
- Sulfur:
- Rhombic sulfur: Stable below 95.5°C.
- Carbon:
| Element | Allotrope | Conditions Favoured |
|---|---|---|
| Carbon | Diamond | High pressure, high temperature |
| Carbon | Graphite | Low pressure, room temperature |
| Phosphorus | White | Low pressure, low temperature |
| Sulfur | Rhombic | Below 95.5°C |
This table illustrates the conditions favouring the formation of specific allotropes for elements like carbon and phosphorus.
Unique conditions can lead to rare allotropes. Amorphous carbon is an example, formed when lacking a crystalline structure. Another rare form is Buckminsterfullerene (C₆₀), found in soot from burning candles.
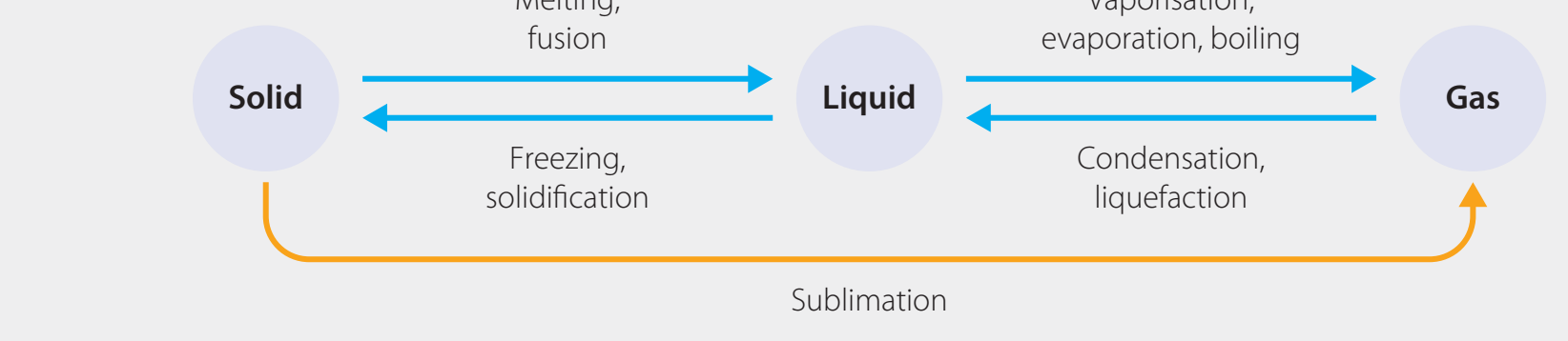 This diagram shows how temperature and pressure affect phase stability.
This diagram shows how temperature and pressure affect phase stability.
Historical Significance
- 1822: Jöns Jakob Berzelius introduced the concept of allotropy, revolutionising chemical understanding.
- Berzelius's terminology enabled systematic chemical studies.
- 1860: Recognition of carbon's allotropes (diamond, graphite) facilitated further exploration into molecular structures.
- This recognition was pivotal for advancements in fields like materials science.
- Engaging Anecdote:
- Berzelius drew inspiration from observing substances like phosphorus and sulfur in varying conditions.
 Highlights key breakthroughs in allotropy.
Highlights key breakthroughs in allotropy.
Comparing Elemental Behaviour
- Reactive Elements:
- Elements such as carbon, sulfur, and phosphorus can have multiple allotropic forms due to their chemical properties.
- Inert Elements:
- Helium and neon are stable due to complete electron shells and do not exhibit allotropy.
Impact on Research and Technology
- Allotropy in Material Science:
- Drives innovation in creating materials with distinct properties.
- Enhances applications in nanotechnology and impacts industrial processes.
- Examples:
- Graphene, an efficient electrical conductor, is transforming the fields of electronics and has potential in medical devices.
Understanding allotropic behaviour is essential for technological advancement. Insights from Graphene present a vision of the future in materials science.
500K+ Students Use These Powerful Tools to Master Carbon's Different Forms For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
197 flashcards
Flashcards on Carbon's Different Forms
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Carbon's Different Forms
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes8 questions
Exam questions on Carbon's Different Forms
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Carbon's Different Forms
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Carbon's Different Forms
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Carbon's Different Forms you should explore
Discover More Revision Notes Related to Carbon's Different Forms to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Intermolecular Forces and Allotropy
Electronegativity and Bond Polarity
305+ studying
195KViews