Photo AI
Last Updated Sep 24, 2025
Polar Molecules Simplified Revision Notes for SSCE HSC Chemistry
Revision notes with simplified explanations to understand Polar Molecules quickly and effectively.
368+ students studying
Polar Molecules
Understanding polar molecules necessitates an exploration of fundamental concepts such as bond polarity, electronegativity, molecular geometry, and dipole moments. These are integral for predicting chemical behaviour.
Bond Polarity and Electronegativity
Bond Polarity
- Definition: Bond Polarity: The unequal sharing of electrons resulting from differences in electronegativity, which causes a variation in electron cloud density and leads to partial charges (δ+ and δ-) on atoms.
Bond Polarity: Unequal electron sharing due to differences in electronegativity, resulting in partial charges.
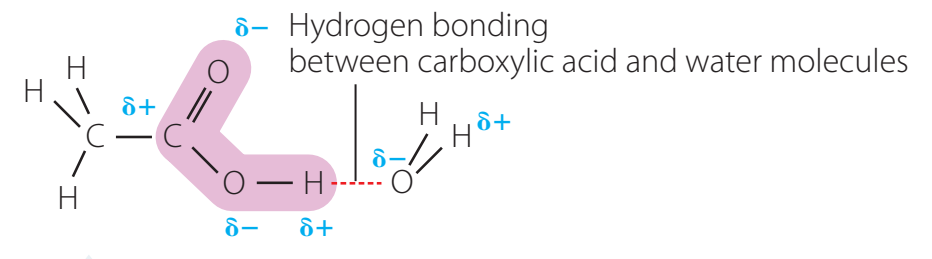
- Example: In the water molecule (H₂O), oxygen is more electronegative, creating a δ- on itself and a δ+ on hydrogen atoms.
Electronegativity
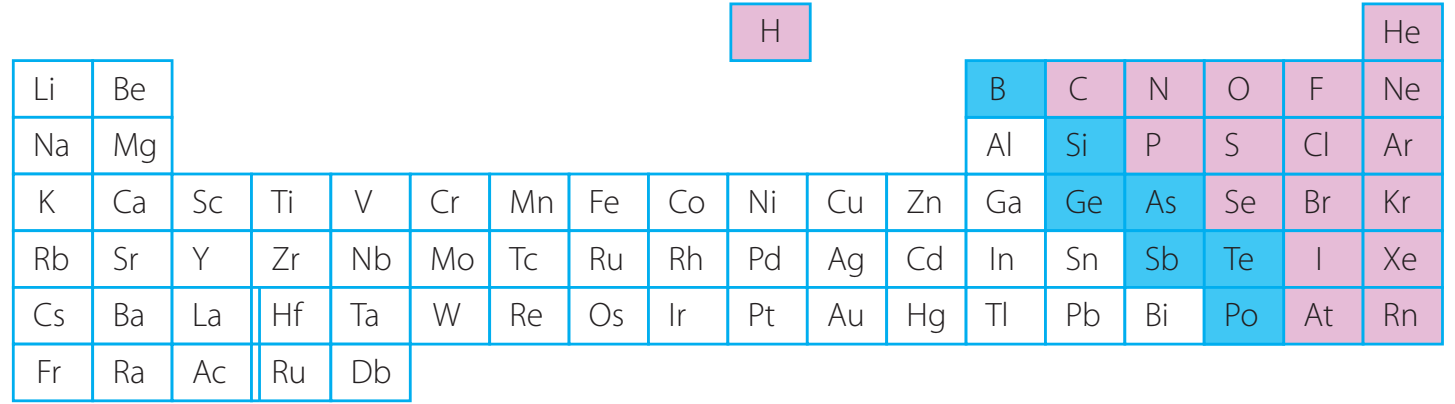
- Definition: Electronegativity: A measure of an atom's ability to attract electrons, which is essential for determining the nature of a bond.
Electronegativity: The ability of an atom to attract electrons, crucial for predicting bond nature.
- Trends:
- Increases from left to right across periods.
- Decreases down groups.
Important Group Trends:
- Group 1 & 2 (Alkali and Alkaline Earth Metals): Possess lower electronegativities.
- Group 17 (Halogens): Exhibit high electronegativities, with fluorine being the most electronegative element.
Polar Covalent Bonds
- These bonds form when there is a considerable difference in electronegativity.
- Examples:
- Hydrogen Chloride (HCl): Exhibits δ+ on H and δ- on Cl.
- Ammonia (NH₃): Displays a dipole moment due to its pyramidal shape.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- VSEPR Theory: Utilised to predict molecular geometries based on interactions among electron pairs.
- It is vital for assessing molecular polarity.
Molecular Geometry & Impact on Polarity
- Shape and Polarity: Bond angles influence polarity. Symmetry may lead to dipole cancellation, resulting in non-polarity.
- Examples:
- Linear (e.g., CO₂): Symmetrical, thus non-polar.
- Bent (e.g., H₂O): Results in a net dipole moment, hence polar.

Symmetry and Asymmetry in Molecules
- Symmetrical structures typically lead to non-polarity, while asymmetrical shapes can induce polarity due to existing dipole moments.
Polar Molecules and Dipole Moments
Polar Molecules
- Characterised by an uneven charge distribution, resulting in a net dipole moment.
Dipole Moments
Dipole Moments: Measure the separation of charge within a molecule, which is critical in interactions with electric fields.
Calculate the Dipole Moment (): where represents the charge and is the distance between charges.
- Features: Dipole moments are measured in Debye units, often determined via spectroscopy.
Visual Representations
- Water (H₂O):
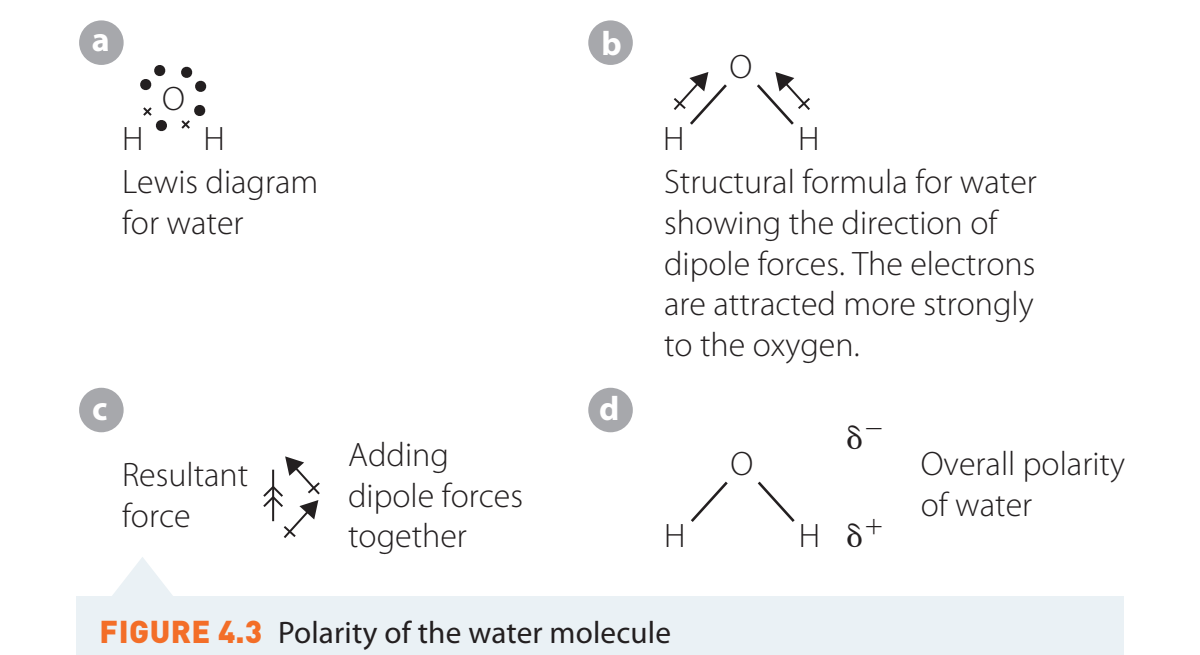
Properties of Polar Molecules
- Interactions:
- Dipole-Dipole: These occur between polar molecules, which causes properties such as higher boiling and melting points.
Dipole-Dipole Interactions: These forces act between polar molecules, akin to magnets attracting each other.
- Boiling and Melting Points: Polar molecules like water demonstrate higher boiling points as a result of stronger intermolecular forces, such as hydrogen bonding.
Solubility
- "Like Dissolves Like": Polar solvents dissolve polar solutes. For example, sugar dissolves in water.

Electrical Conductivity
- Certain polar solutions, like saline, conduct electricity owing to the presence of ions.
Practice Questions with Solutions
-
Question: What effect does the electron cloud distribution have on molecular polarity? Solution: Uneven electron cloud distribution creates partial charges (δ+ and δ-) on atoms, resulting in a polar molecule. The greater the electronegativity difference, the more pronounced the polarity.
-
Question: Calculate the difference in electronegativity between C (2.5) and O (3.5) and determine if their bond is polar. Solution: Electronegativity difference = 3.5 - 2.5 = 1.0. Since the difference is between 0.5 and 1.7, the C-O bond is considered moderately polar.
500K+ Students Use These Powerful Tools to Master Polar Molecules For their SSCE Exams.
Enhance your understanding with flashcards, quizzes, and exams—designed to help you grasp key concepts, reinforce learning, and master any topic with confidence!
197 flashcards
Flashcards on Polar Molecules
Revise key concepts with interactive flashcards.
Try Chemistry Flashcards18 quizzes
Quizzes on Polar Molecules
Test your knowledge with fun and engaging quizzes.
Try Chemistry Quizzes8 questions
Exam questions on Polar Molecules
Boost your confidence with real exam questions.
Try Chemistry Questions27 exams created
Exam Builder on Polar Molecules
Create custom exams across topics for better practice!
Try Chemistry exam builder24 papers
Past Papers on Polar Molecules
Practice past papers to reinforce exam experience.
Try Chemistry Past PapersOther Revision Notes related to Polar Molecules you should explore
Discover More Revision Notes Related to Polar Molecules to Deepen Your Understanding and Improve Your Mastery
96%
114 rated
Intermolecular Forces and Allotropy
Carbon's Different Forms
252+ studying
199KViews96%
114 rated
Intermolecular Forces and Allotropy
Electronegativity and Bond Polarity
218+ studying
193KViews